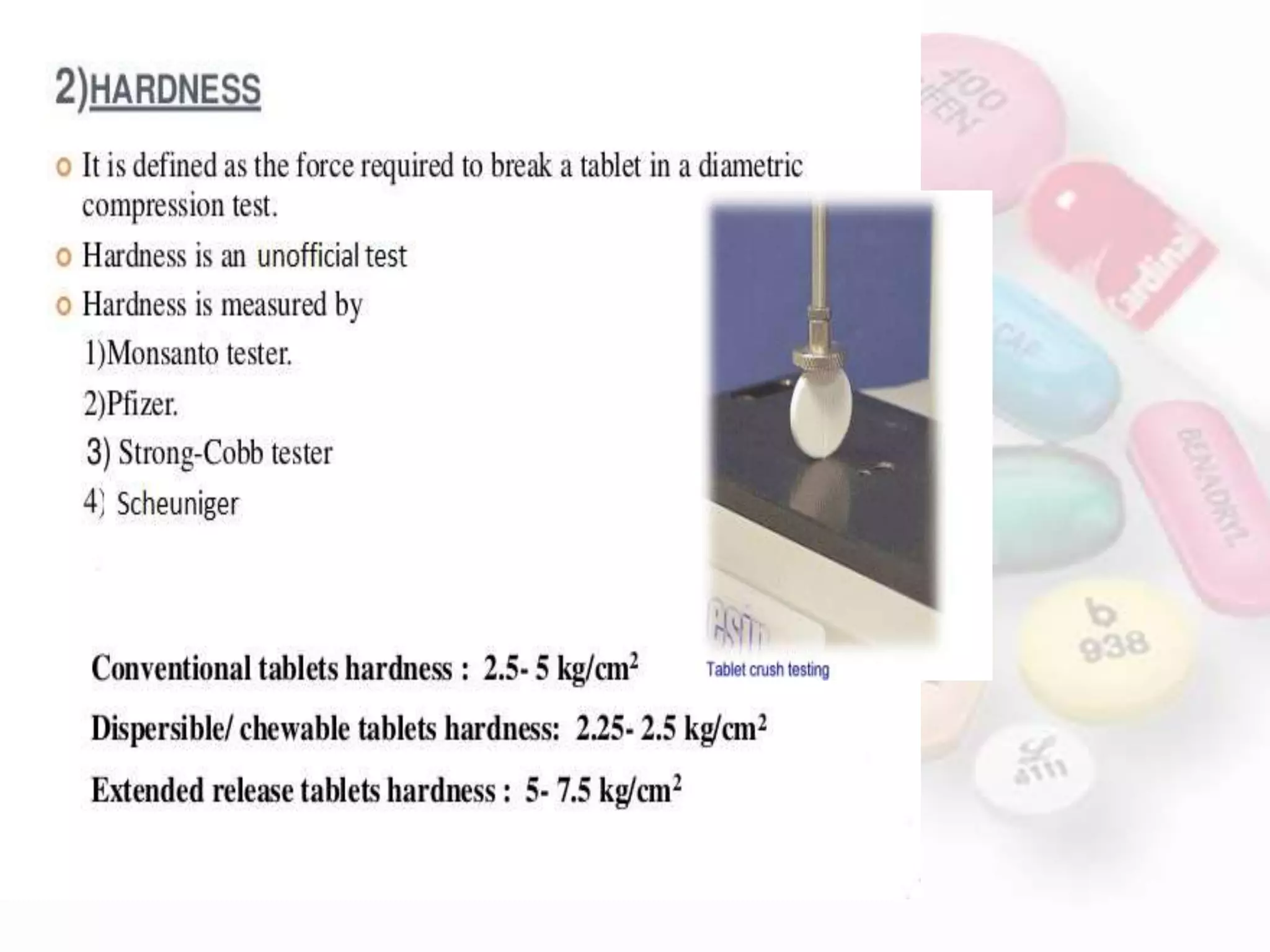
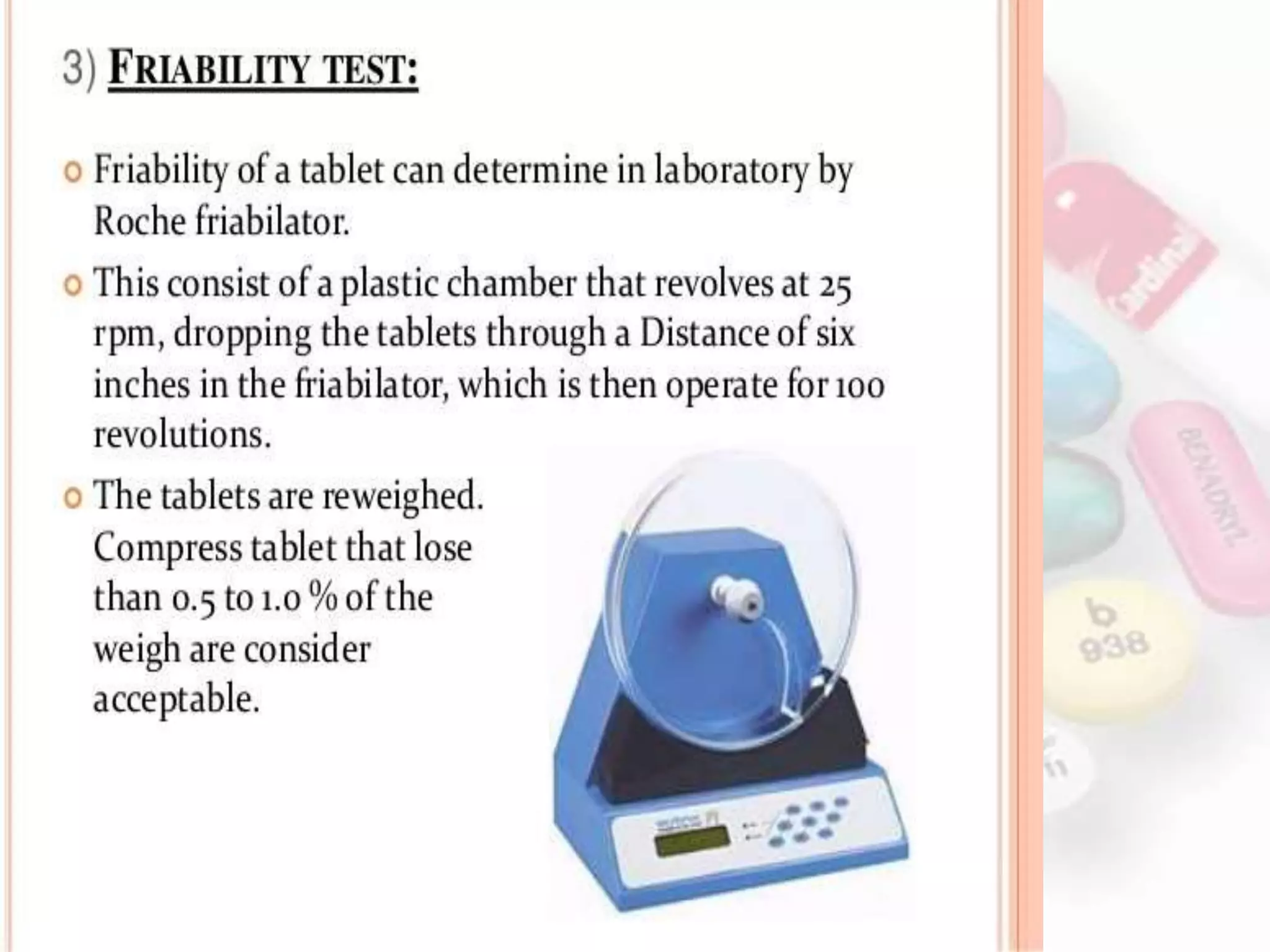
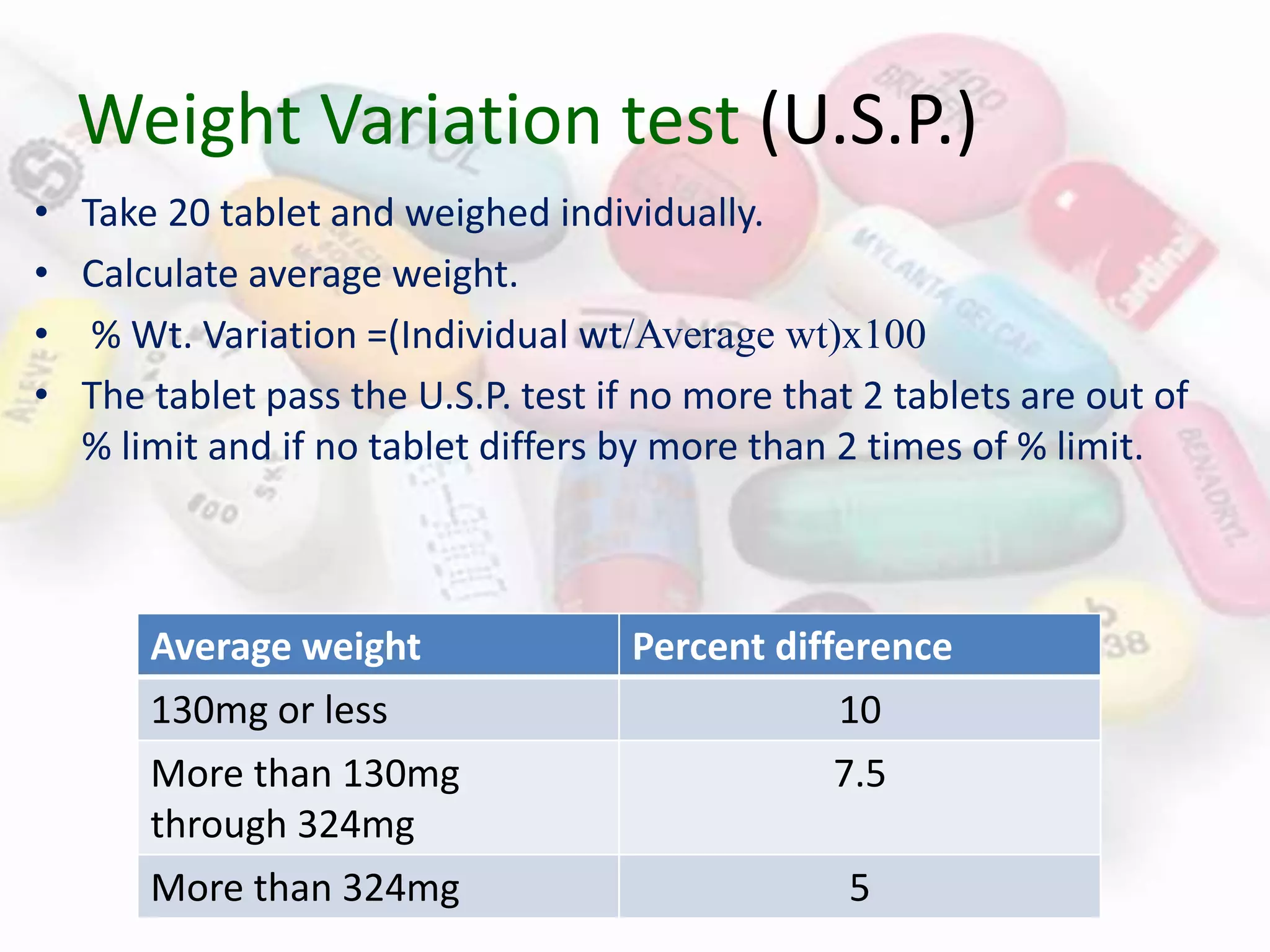
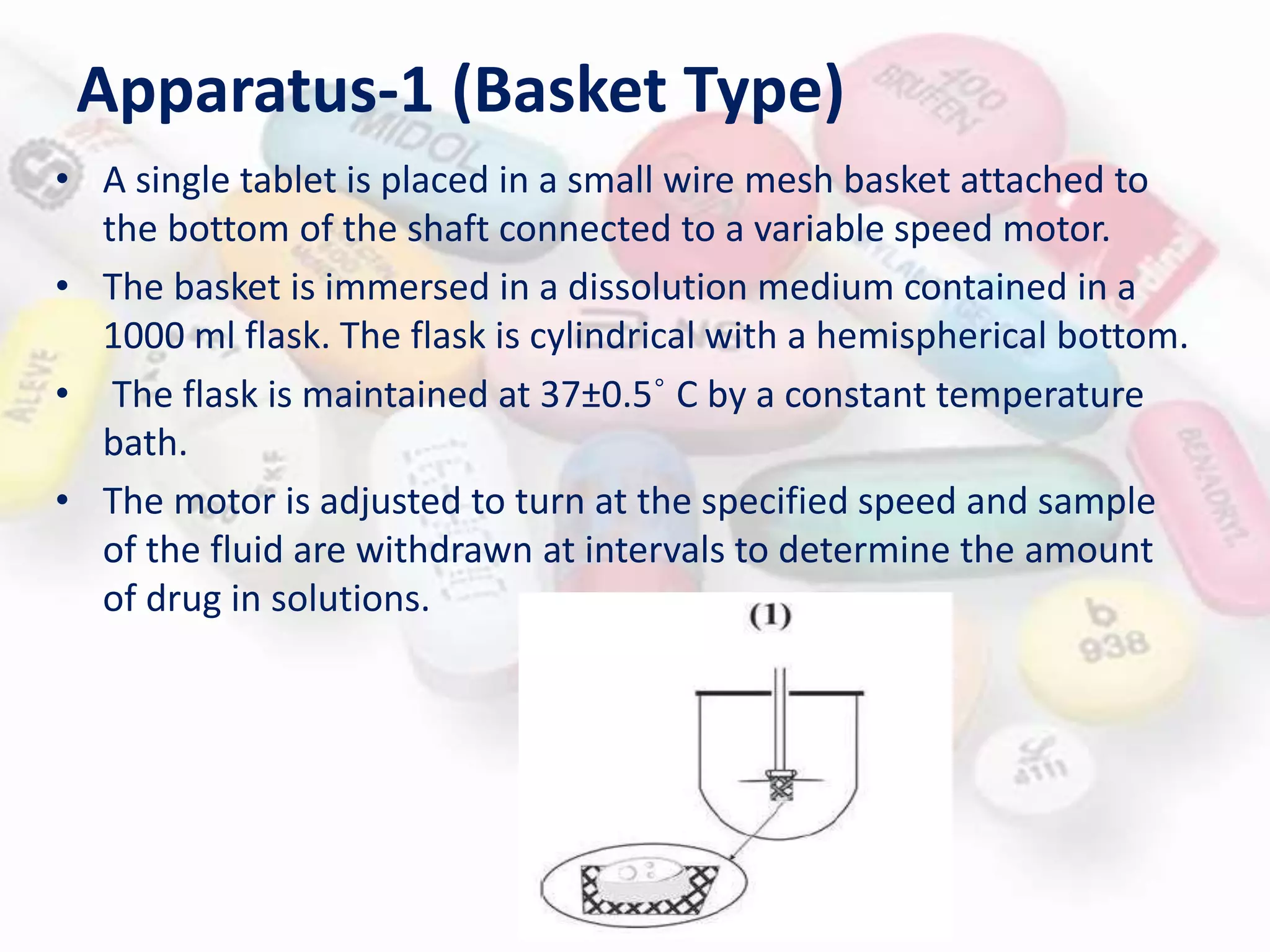
This document evaluates various properties of tablets including size, shape, thickness, friability, weight variation, disintegration, and dissolution. It describes common tests used to analyze these properties, including friability testing to measure how easily tablets crumble, weight variation testing to check consistency of tablet weights, disintegration testing using glass tubes in fluid, and dissolution testing using apparatus 1 (basket type) or apparatus 2 (paddle type) to measure how quickly drugs dissolve. The document provides details on procedures, equipment, and standards used for these pharmaceutical quality control tests.