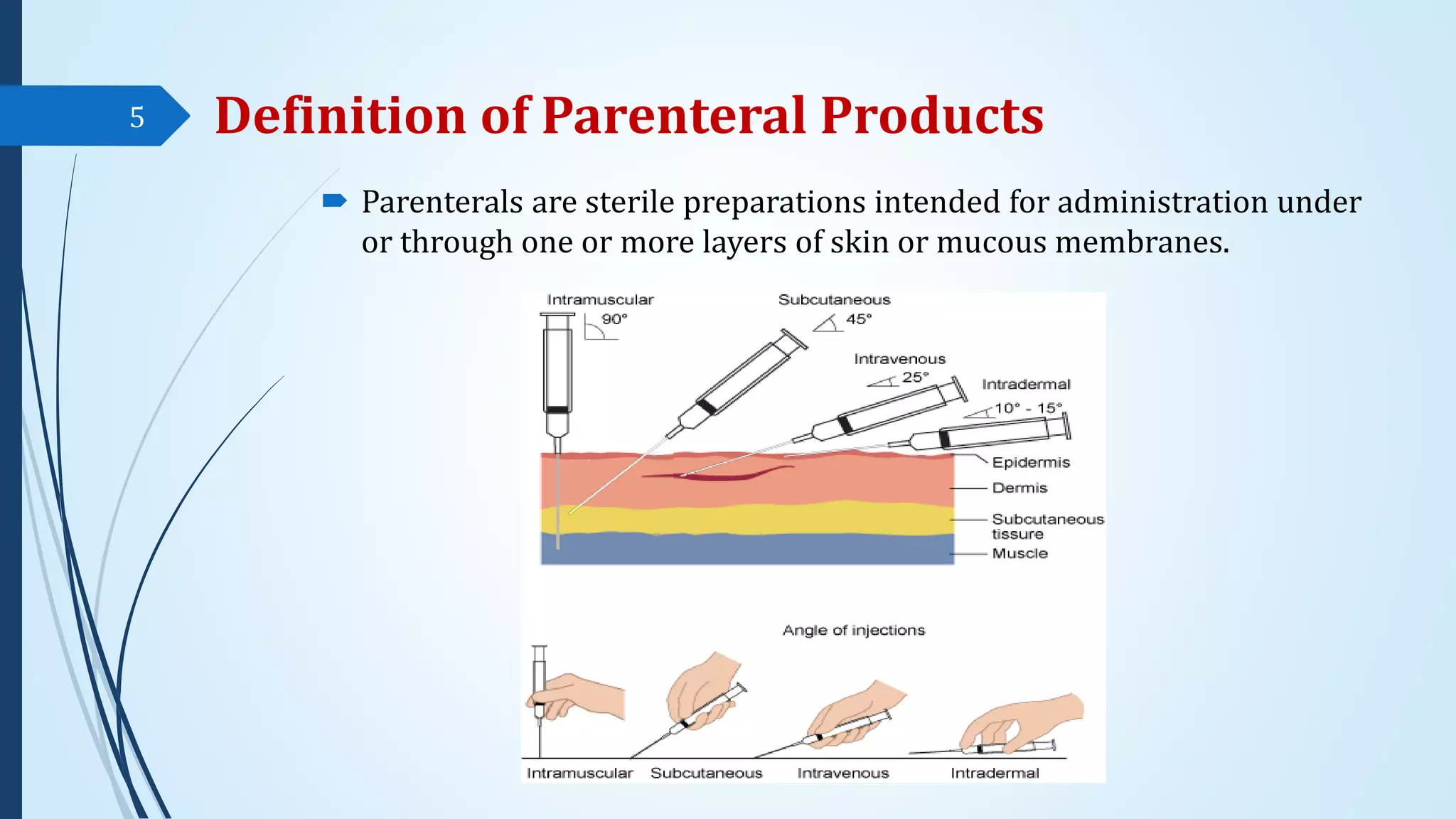
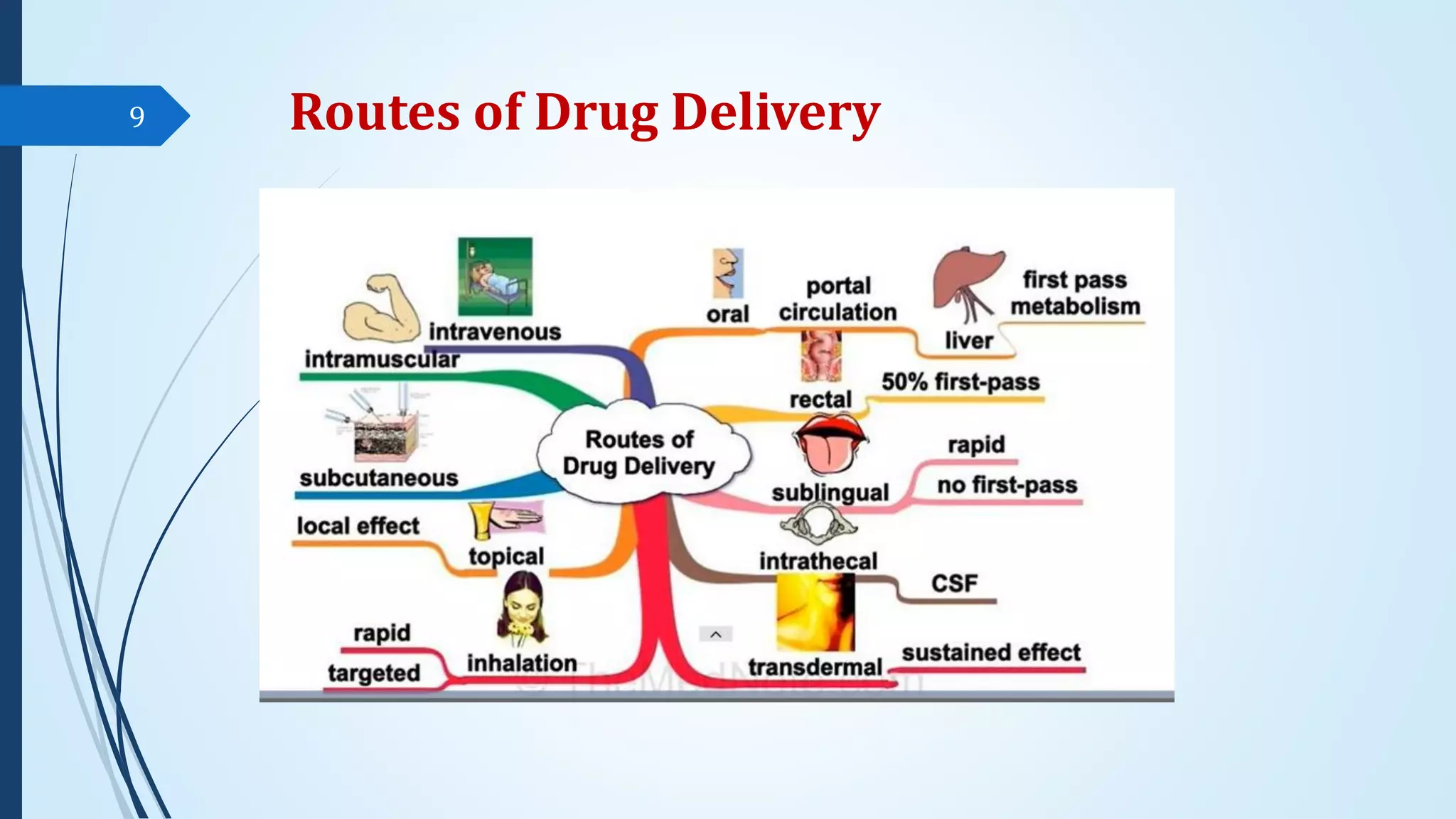
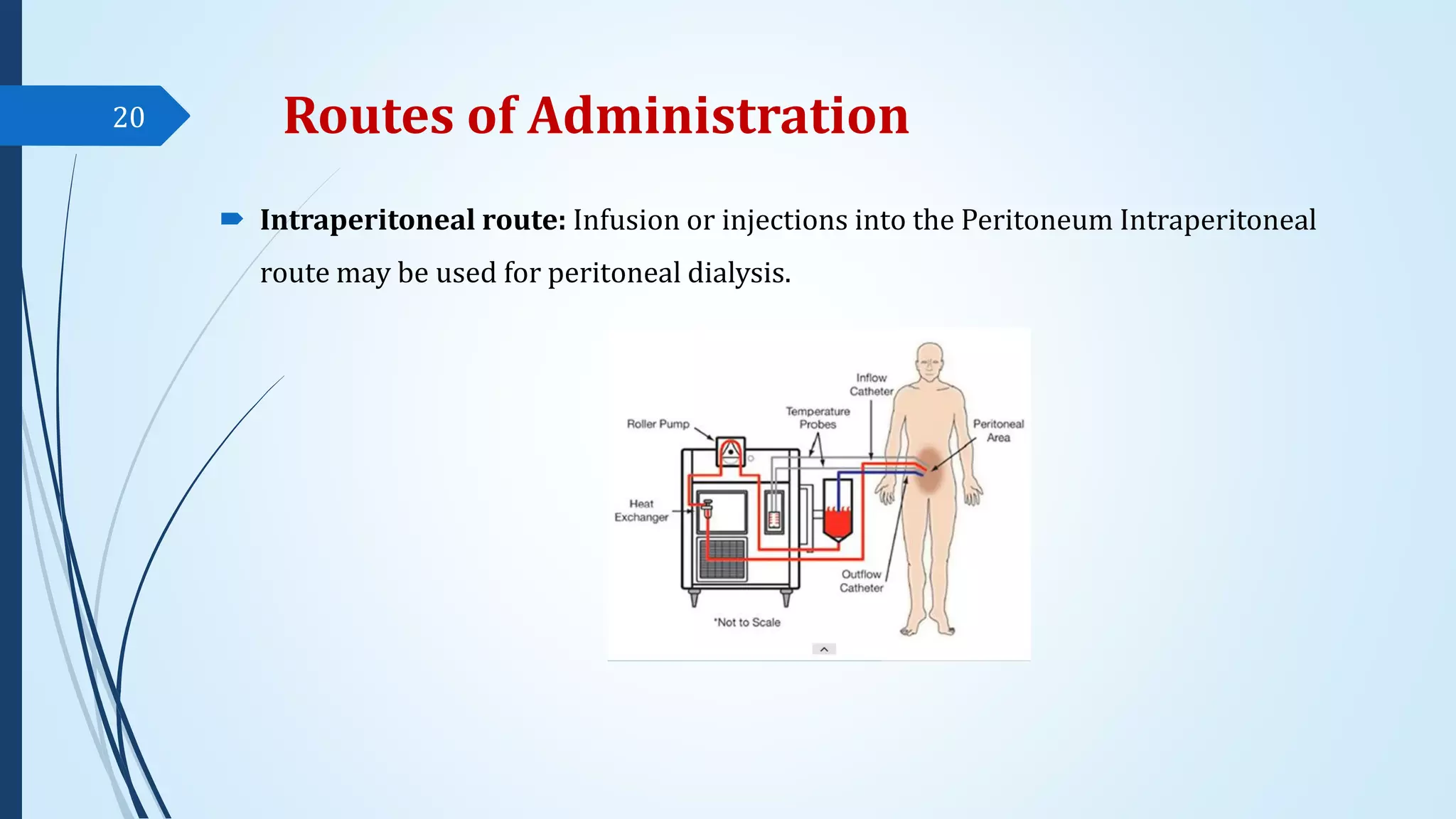
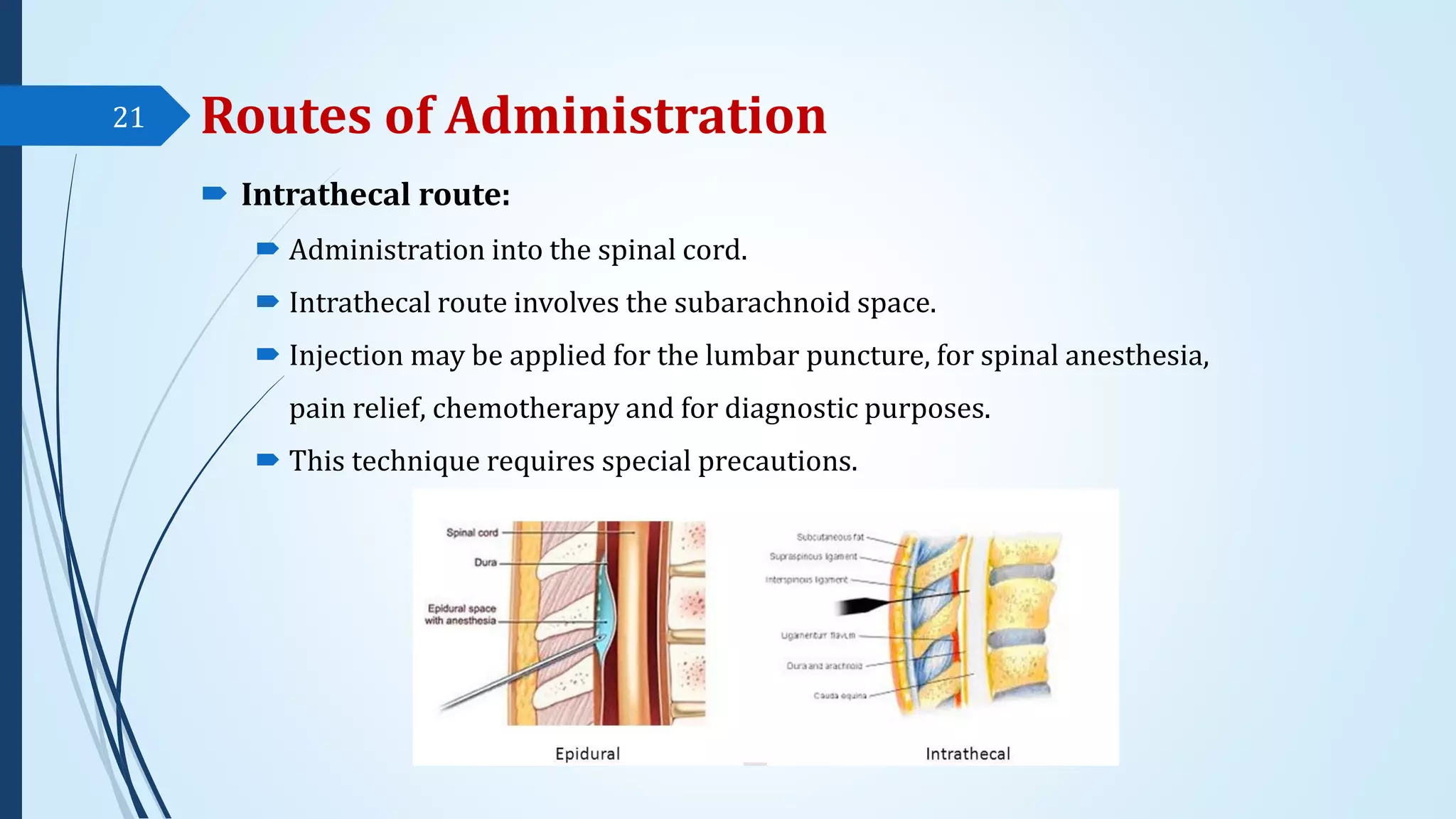
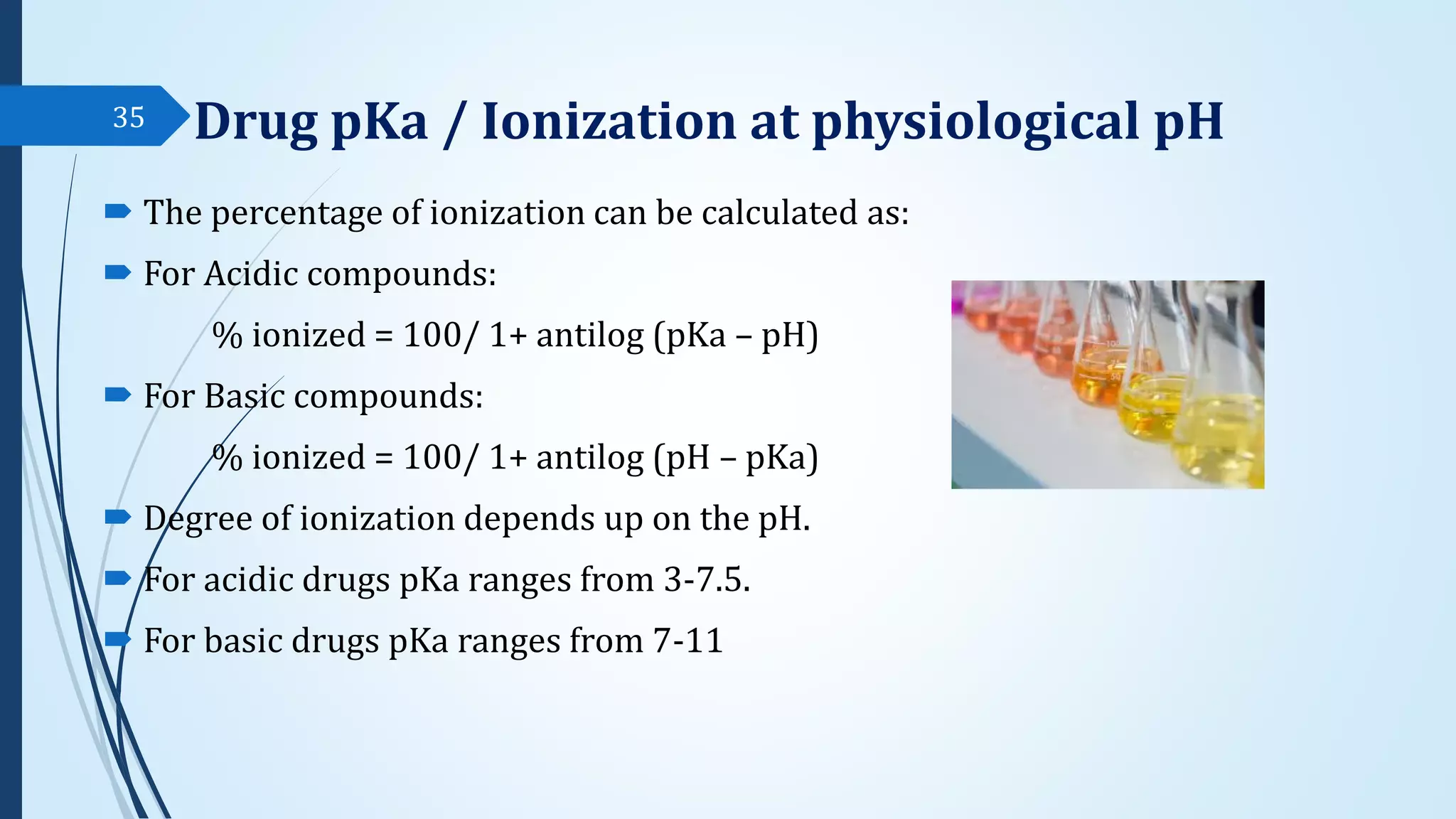
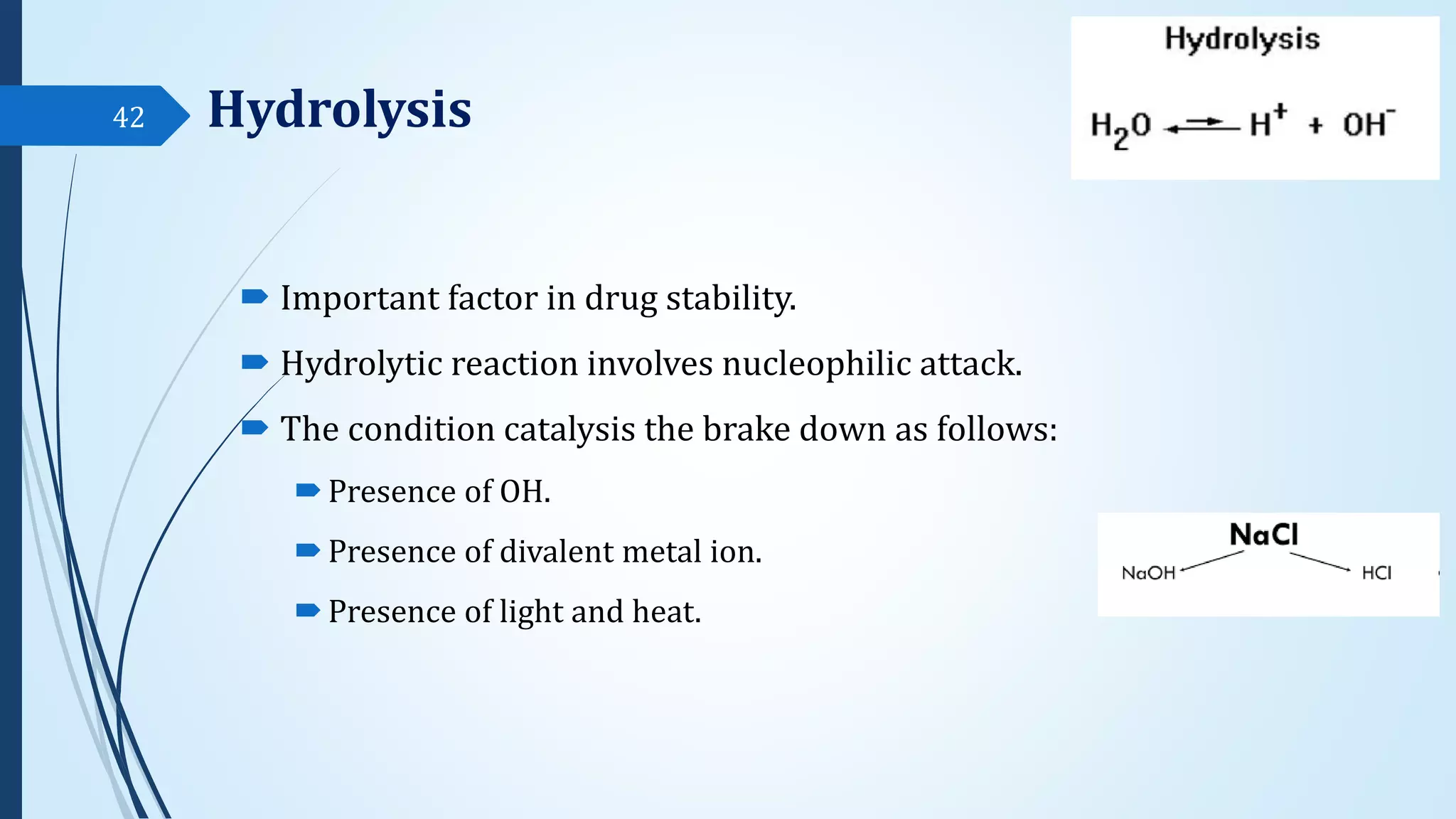
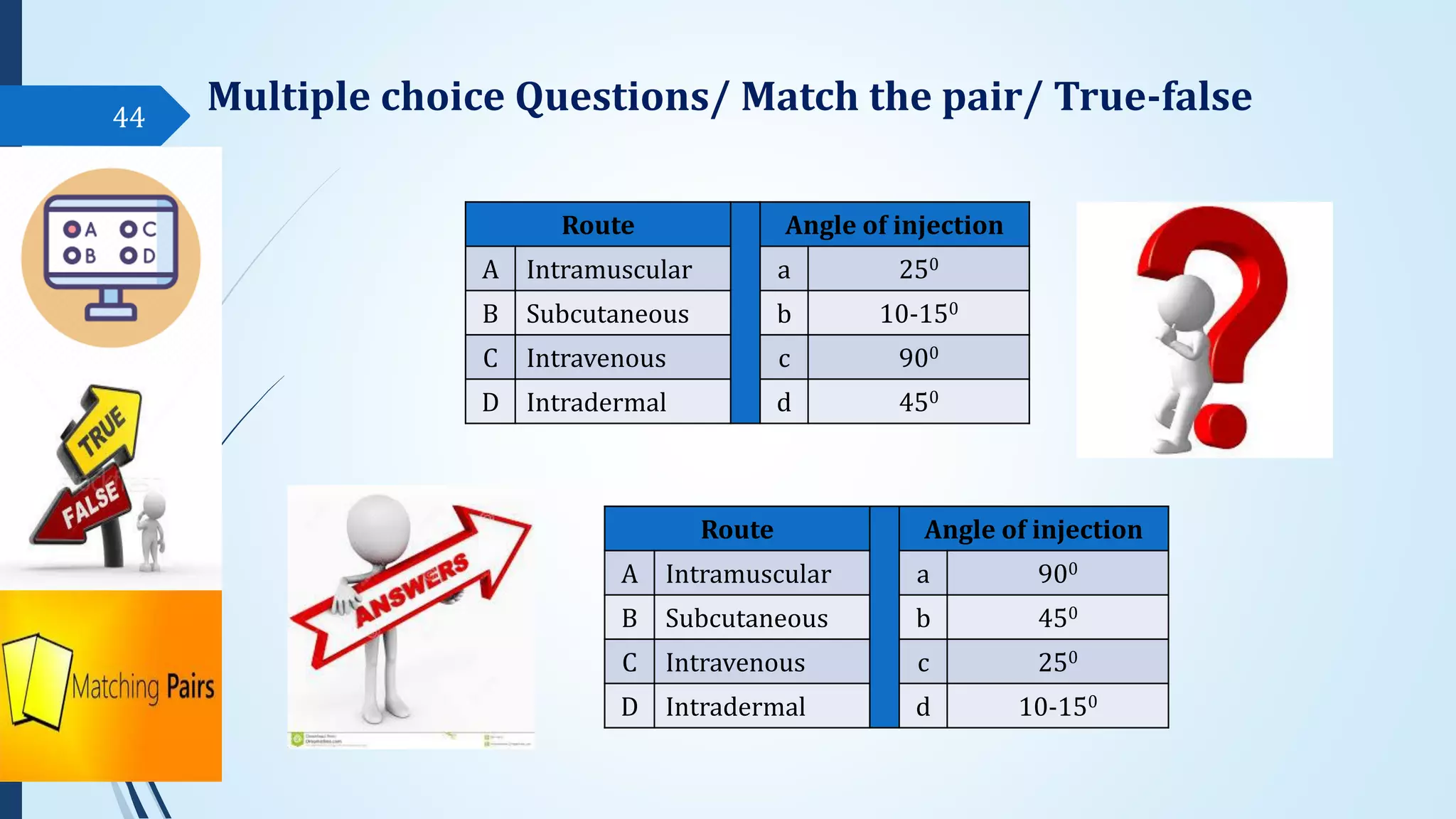
The document discusses sterile formulations and parenteral products. It covers key topics like routes of parenteral administration, pre-formulation of sterile products, physicochemical properties, general requirements, and sterility testing. The different routes discussed are subcutaneous, intramuscular, intravenous, and others. Ideal properties of sterile dosage forms include sterility, isotonicity, being pyrogen-free and particle-free. The document also differentiates between small volume parenterals and large volume parenterals.