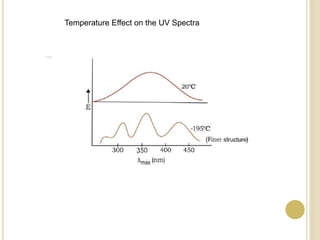
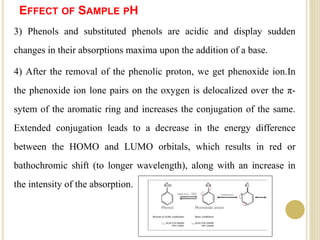
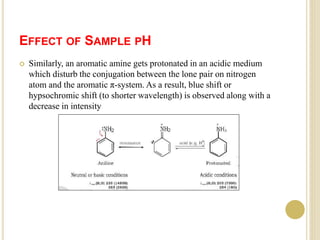
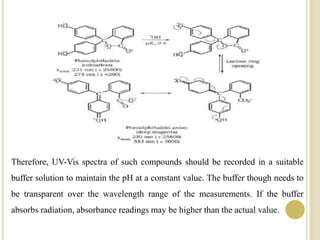
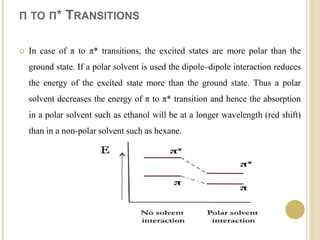
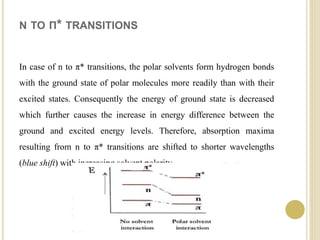
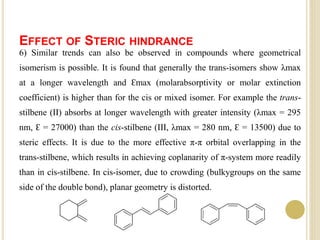
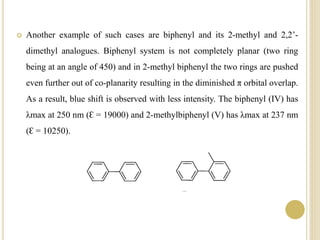
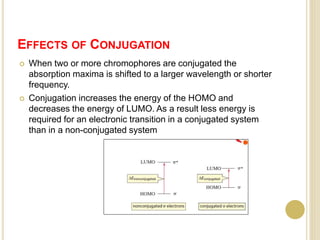
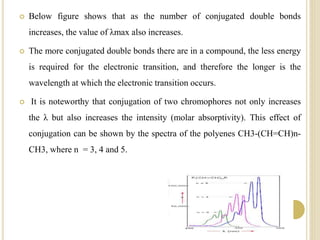
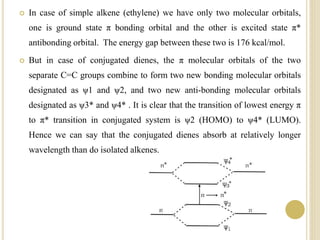
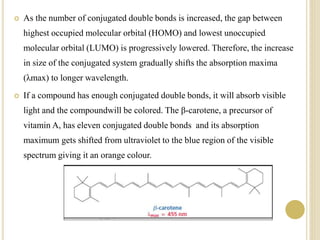
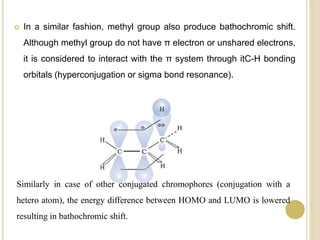
This document discusses several factors that can affect UV-Vis absorption spectra, including sample temperature, concentration, pH, solvent, and molecular structure. Lowering the temperature results in sharper, more defined absorption bands. Increasing the concentration can lead to band broadening at high levels due to molecular interactions. Changing the pH can cause shifts in absorption maxima for compounds like phenols and anilines. The solvent can also impact absorption by stabilizing different electronic states to varying degrees. Molecular structure factors such as conjugation, steric hindrance, and isomerism further influence absorption spectra.