This document provides an overview of different ionization techniques used in mass spectrometry. It discusses gas phase ionization methods like electron impact and chemical impact ionization. It also covers desorptive ionization techniques such as field desorption, fast atom bombardment, and matrix-assisted laser desorption ionization. Finally, it summarizes evaporative ionization methods like atmospheric pressure chemical ionization and atmospheric pressure photoionization. The document aims to explain the basic principles, applications, advantages, and limitations of various ionization methods for mass spectrometry analysis.




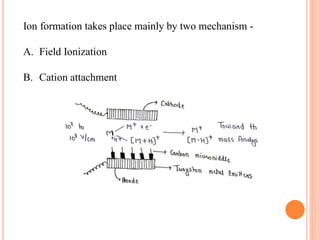
![2) Fast atom bombardment [FAB]
•It is a soft ionization method classified under desorption method.
•FAB is used to determine the molecular weight of the
Compound having the Size from 300 to 6000 Daltons-.
•Generally used to determine molecular weight of Peptides.
•Methodology:-](https://image.slidesharecdn.com/akshaydholempat-230318054621-954a5004/85/Ionization-Techniques-In-Mass-Spectroscopy-14-320.jpg)

![Evaporative Ionization
1) Atmospheric Pressure chemical Ionization [APCI]
It is a type of soft Ionization technique based on the mechanism
of evaporation and Carried out the atmospheric Pressure.
Actually APCI is Combination of chemical Ionization [CI] and
electrospray Ionization (ESI) with Some deviation.](https://image.slidesharecdn.com/akshaydholempat-230318054621-954a5004/85/Ionization-Techniques-In-Mass-Spectroscopy-20-320.jpg)
![2) Atmospheric Pressure Photo Ionization [APPI]
• It is a type of soft ionization technique.
• Based on the mechanism of evaporation and Carried
out at atmospheric Pressure.
• APPI is Similar to APCI but ionization is APPI is due
to photons generated by UV.](https://image.slidesharecdn.com/akshaydholempat-230318054621-954a5004/85/Ionization-Techniques-In-Mass-Spectroscopy-21-320.jpg)
