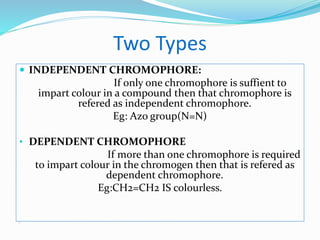
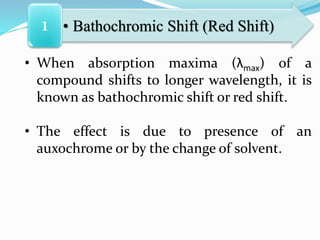
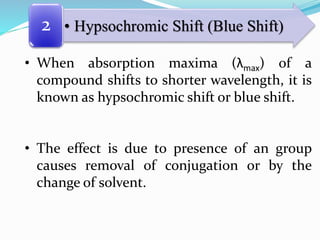
This document discusses chromophores and how solvents affect absorption spectra. It defines a chromophore as a covalently bonded group that absorbs UV or visible radiation. Chromophores are classified as independent or dependent based on the number needed to impart color. Absorption maxima can shift to longer (bathochromic) or shorter (hypsochromic) wavelengths due to auxochromes or solvent changes. Solvent polarity also affects absorption based on the type of electronic transition involved. Temperature and solvent interactions determine the fineness of absorption bands.