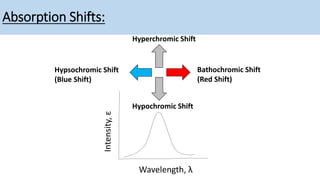
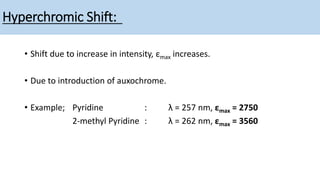
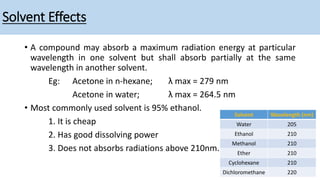
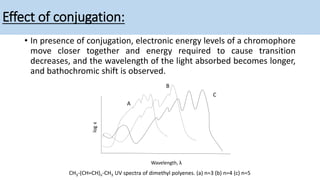
The document discusses the factors affecting absorption in chromophores and auxochromes, elaborating on their definitions, types, and how they interact with light. It explains absorption shifts such as bathochromic and hypsochromic shifts and the influences of solvent polarity on absorption wavelengths. Additionally, it explores the impact of conjugation on electronic energy levels and consequent changes in light absorption.