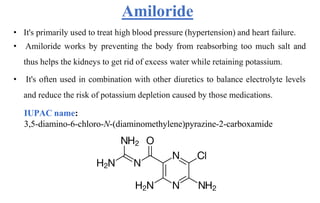
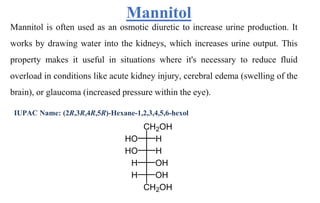
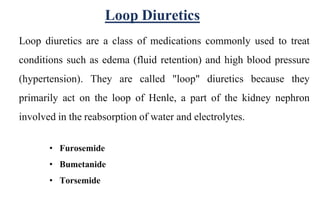
Diuretics are chemicals that increase urine formation and are used to treat conditions like hypertension and edema through increased excretion of electrolytes and water. They include various classifications such as osmotic, thiazide, loop, and potassium-sparing diuretics, each with different mechanisms of action and uses. These drugs can have side effects, including electrolyte imbalances, dehydration, and specific drug-related complications.







![Benzthiazide
Benzthiazide is used to treat hypertension and edema. Like other thiazides,
benzthiazide promotes water loss from the body (diuretics). They inhibit
Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys.
IUPAC name 6-chloro-1,1-dioxo-3-(phenylmethylsulfanylmethyl)- 4H-benzo[e][1,2,4]
thiadiazine-7-sulfonamide](https://image.slidesharecdn.com/diuretics-241126055651-039bd5d0/85/Diuretics-Medicinal-Chemistry-20-320.jpg)


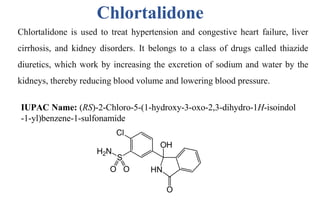

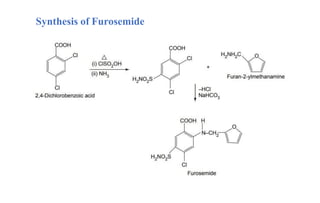
![Furosemide
• Furosemide is used to reduce extra fluid in the body (edema) caused by
conditions such as heart failure, liver disease and kidney disease.
• They are organic compounds containing a benzenesulfonamide with an
amine group attached to a benzene ring.
IUPAC Name: 4-Chloro-2-[(furan-2-ylmethyl)amino]-5-sulfamoylbenzoic acid](https://image.slidesharecdn.com/diuretics-241126055651-039bd5d0/85/Diuretics-Medicinal-Chemistry-27-320.jpg)

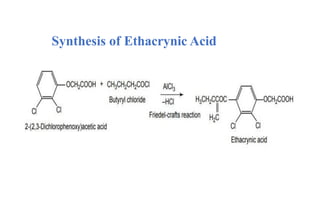
![Ethacrynic Acid
Ethacrynic acid is a medication primarily used to treat fluid retention (edema) in
individuals with congestive heart failure, liver disease, or kidney problems. It
belongs to a class of drugs called loop diuretics, which work by increasing the
elimination of sodium and water from the body through the urine.
IUPAC name: [2,3-dichloro-4-(2-methylenebutanoyl)phenoxy]acetic acid](https://image.slidesharecdn.com/diuretics-241126055651-039bd5d0/85/Diuretics-Medicinal-Chemistry-31-320.jpg)


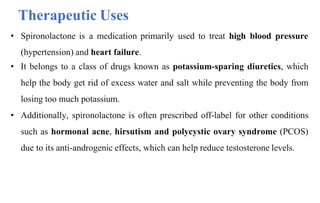
![Spiranolactone
• Spironolactone is a medication primarily used to treat fluid build-up due to
heart failure, liver scarring, or kidney disease.
• It's also used for treating high blood pressure and in certain cases of hormonal
acne in women.
IUPAC name
S-[(7R,8R,9S,10R,13S,14S,17R)-10,13-Dimethyl-3,5'-dioxospiro[2,6,7,8,9,11,12,14,15,16-decahydro-1H
-cyclopenta[a]phenanthrene-17,2'-oxolane]-7-yl] ethanethioate](https://image.slidesharecdn.com/diuretics-241126055651-039bd5d0/85/Diuretics-Medicinal-Chemistry-36-320.jpg)