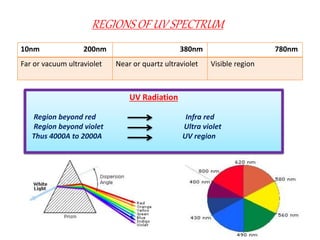
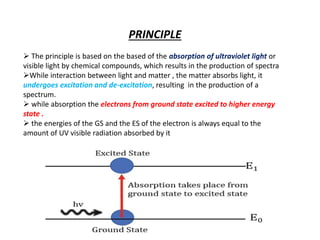
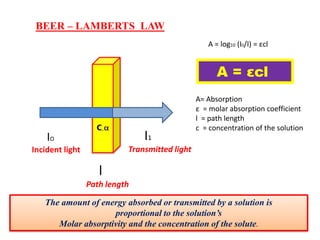
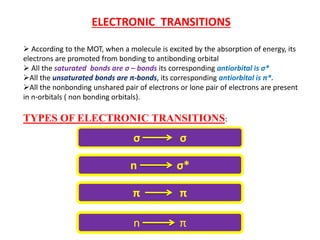
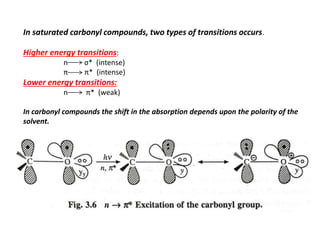
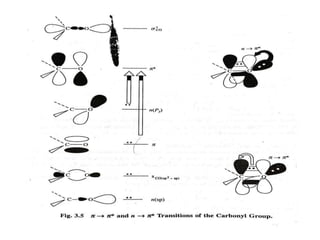
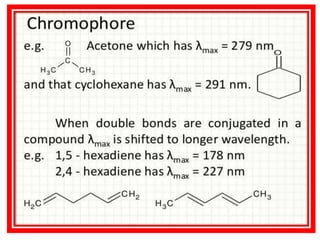
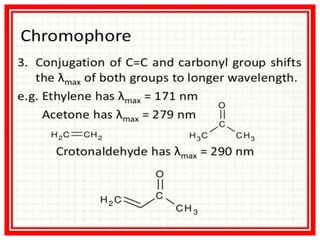
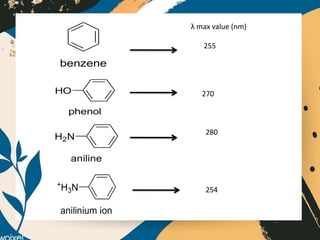
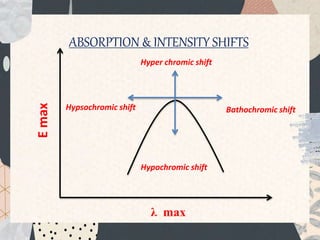
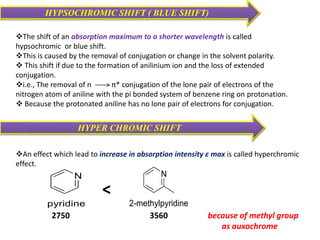
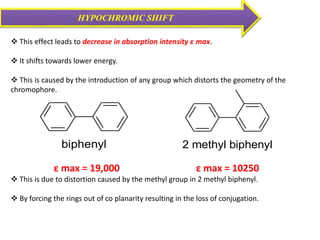
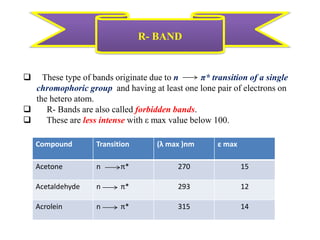
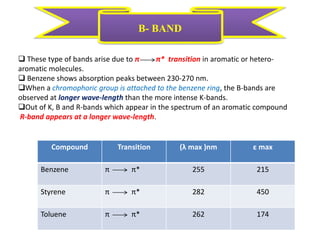
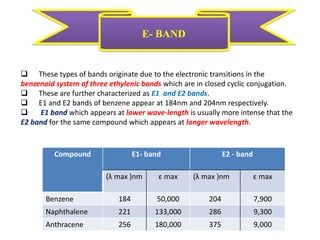
UV-visible spectroscopy studies the interaction of ultraviolet and visible light with matter, focusing on electronic transitions due to energy absorption. It involves various types of electronic transitions such as σ σ*, n σ*, and π π*, dependent on the structure of the molecules and their chromophores. Additionally, it addresses the effects of auxochromes on absorption characteristics and different shifts in wavelengths caused by solvent polarity and molecular interactions.