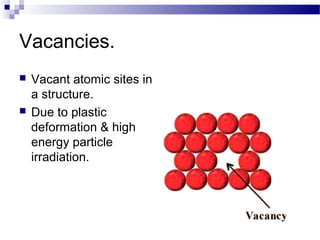
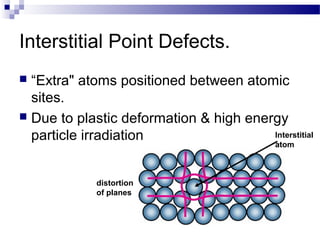
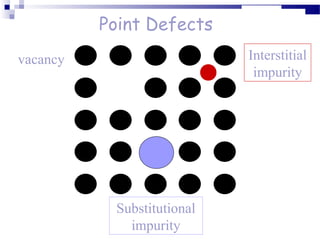
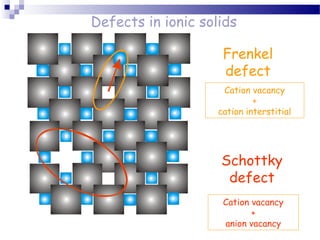
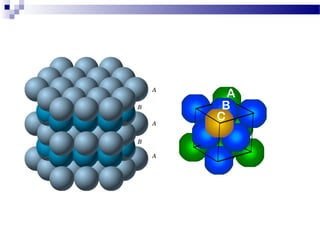
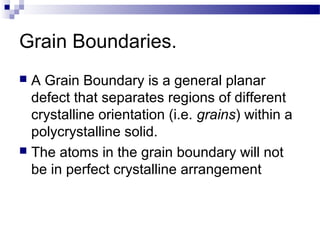
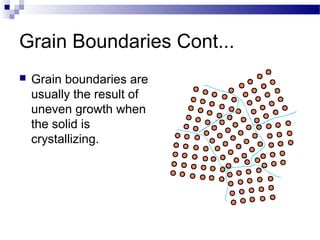
There are several types of defects that can occur in crystalline materials, including point defects, line defects, and grain boundaries. Point defects include vacancies, interstitial atoms, and substitutional/interstitial impurities. Line defects include stacking faults which occur when the regular stacking sequence of atomic planes is disturbed. Grain boundaries separate crystalline grains of different orientations in polycrystalline materials. These defects influence many properties of materials.