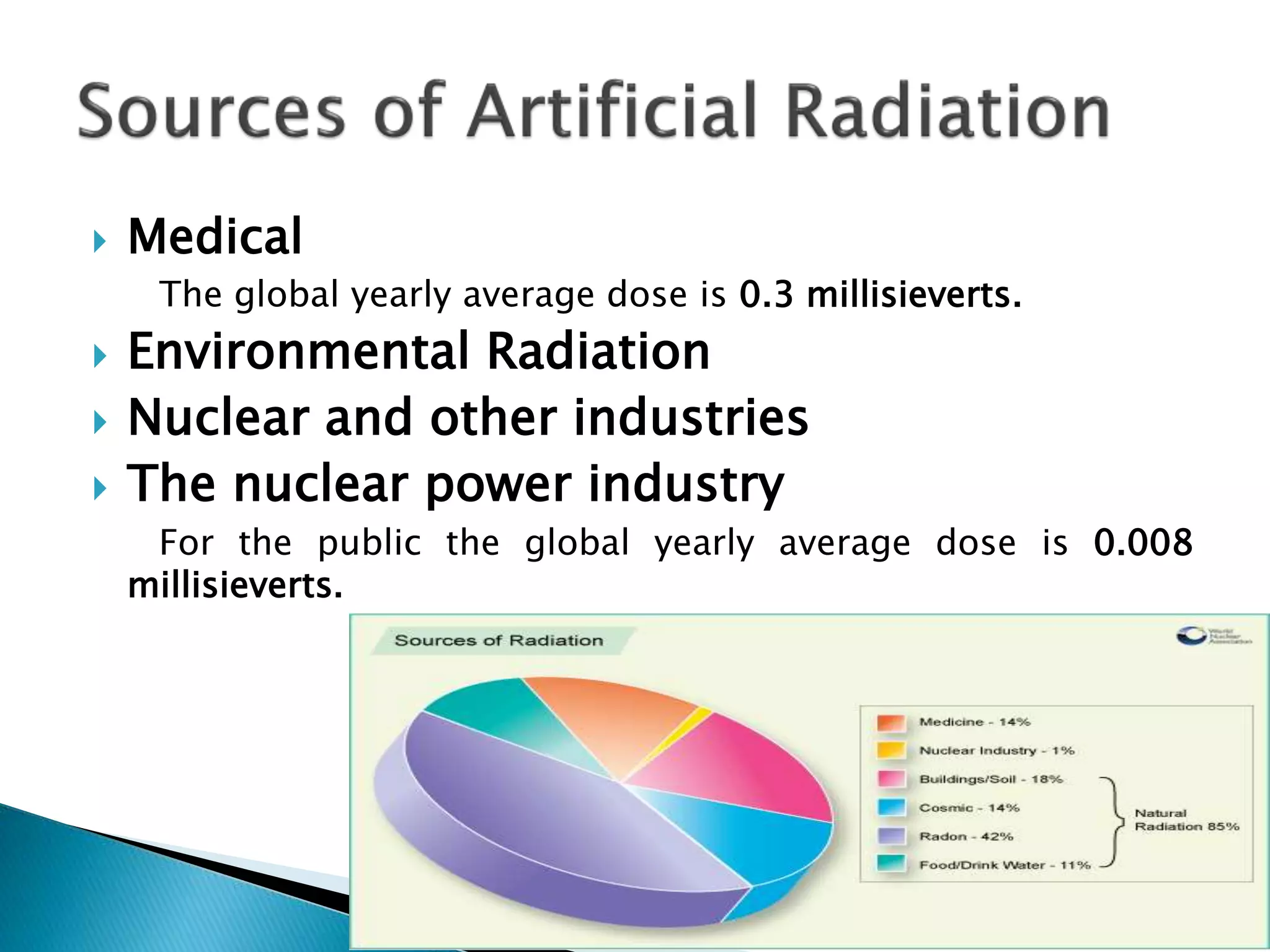
Radiation is energy transmitted through space or matter in the form of waves or particles. It includes visible light, ultraviolet light from the sun, and radio/TV signals. Nuclear radiation comes from unstable atoms undergoing radioactive decay, emitting particles like alpha and beta or electromagnetic waves like gamma rays. Exposure to ionizing radiation can damage living tissue. Natural sources of radiation include cosmic rays, radioactive elements in the earth's crust like radon, and some food/drink. Medical procedures and occupational exposures also contribute. In materials, radiation can cause impurities from nuclear reactions, ionization by charged particles, and displacement of atoms from their normal positions in the crystal structure.