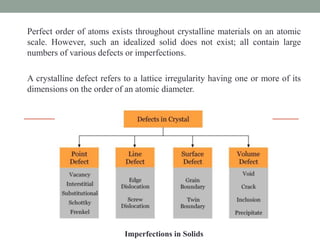
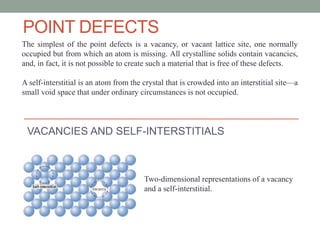
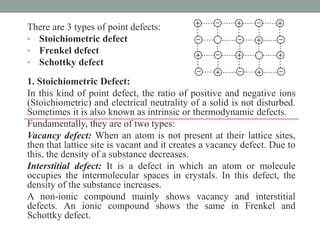
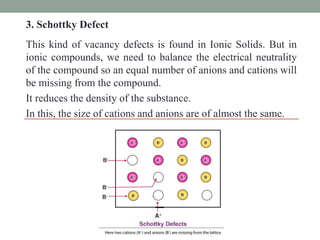
This document discusses different types of point defects that can occur in crystalline solids. There are three main types of point defects: 1) Stoichiometric defects include vacancies where an atom is missing from its lattice site, and interstitials where an atom occupies an interstitial space in the lattice. 2) Frenkel defects occur when a smaller cation moves from its lattice site into an interstitial space, creating both a vacancy and interstitial. 3) Schottky defects occur in ionic solids and involve an equal number of cation and anion vacancies to maintain charge neutrality, reducing the material's density.