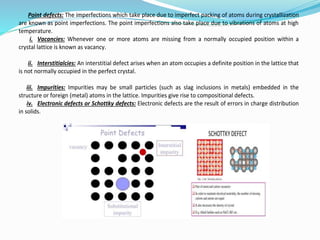
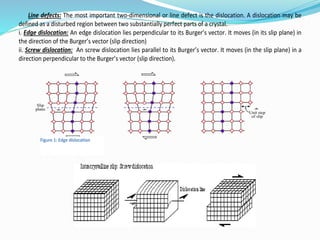
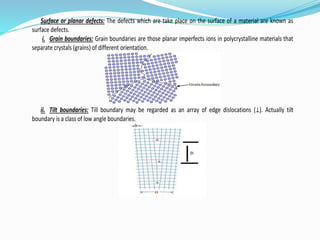
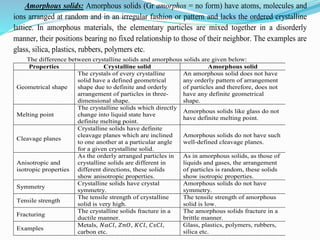
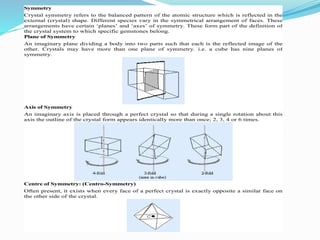
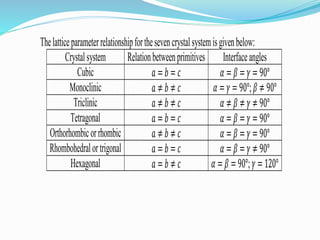
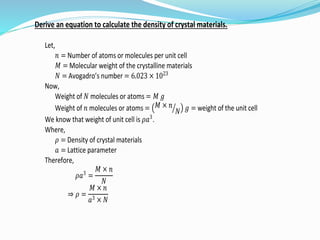
The document discusses different types of solids and their crystal structures. It defines crystalline and amorphous solids. Crystalline solids have an orderly arrangement of atoms and distinct crystal structures, while amorphous solids lack any orderly pattern. Common crystalline structures include face-centered cubic, body-centered cubic, and hexagonal close-packed. These structures differ in how atoms are arranged within the unit cell. The document also discusses properties like atomic radius, packing efficiency, and symmetry elements of different crystal structures.









![Bragg’s equation: In 1913 the father-and-son, William Lawrence Bragg and William Henry Bragg
worked out a mathematical relation to determine interatomic distances from X-ray diffraction
patterns. This relation is called the Bragg’s equation. They showed that:
i. The X-ray diffracted from atoms in crystal planes obeys the laws of reflection.
ii.The two rays reflected by successive planes will be in phase if the extra distance travelled by
the second ray is an integral number of wavelengths.
The equation is
𝑛𝜆 = 2𝑑 sin 𝜃
Where,
𝜃 = diffraction angle
𝜆 = wave length of the X-ray
𝑑 = distance between the planes
Derivation: Let a beam of X-rays is falling on the crystal surface. Two successive atomic planes of
the crystal are shown separated by a distance 𝑑. Let the X-rays of wavelength 𝜆 strikes the planes at an
angle 𝜃. For the first plane, 𝐴𝐵 is incident ray and 𝐵𝐶 is reflected ray. For the second plane, 𝐷𝐸 is
incident ray and 𝐸𝐹 is reflected ray. Now we draw perpendicular lines 𝐵𝐺 and 𝐵𝐻 on 𝐷𝐸 and 𝐸𝐹
respectively.
After falling on first plane some of the rays will be reflected at the same angle. Some of the rays
will penetrate and get reflected from the second plane. These rays will reinforce those reflected from
the first plane if the extra distance travelled by them (𝐺𝐸 + 𝐸𝐻) is equal to integral number, 𝑛, of
wavelengths. That is,
𝑛𝜆 = 𝐺𝐸 + 𝐸𝐻 ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ ⋯ (1)
Geometry shows that
𝐺𝐸 = 𝐸𝐻 = 𝐵𝐸 sin 𝜃
From equation (1), we get,
𝑛𝜆 = 𝐵𝐸 sin 𝜃 + 𝐵𝐸 sin 𝜃
⇒ 𝑛𝜆 = 𝑑 sin 𝜃 + 𝑑 sin 𝜃
⇒ 𝑛𝜆 = 2𝑑 sin 𝜃
This is Bragg’s equation.
[Derived]](https://image.slidesharecdn.com/crystrstalautosaved-180421170245/85/Crystal-Structure-BCC-FCC-HCP-15-320.jpg)