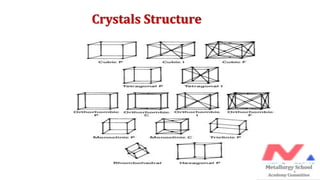
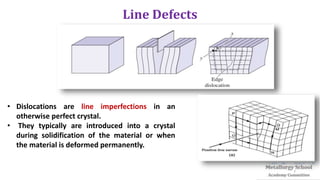
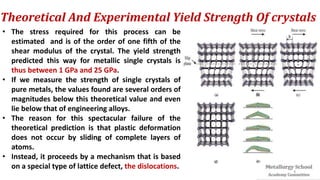
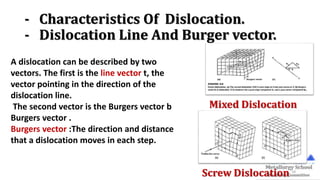
The document presents foundational concepts in metallurgy, focusing on crystalline and amorphous materials, crystal defects, and the mechanisms of plastic deformation. It discusses dislocations, their types, and how they influence the mechanical properties of materials, particularly in relation to yield strength and slipping mechanisms. The text also explores theories related to work hardening and the impact of solute atoms on dislocation behavior.