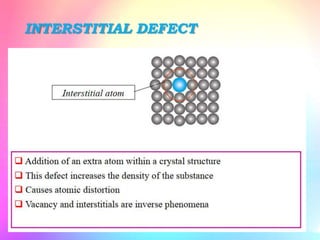
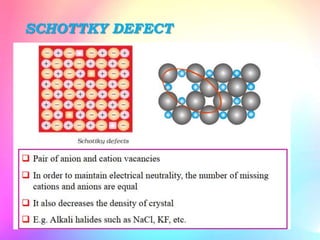
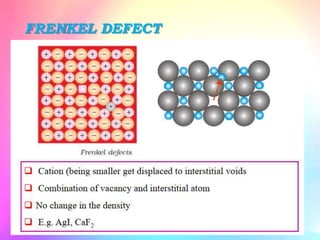
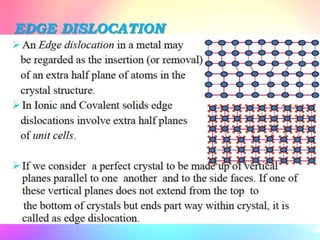
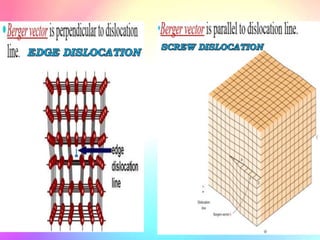
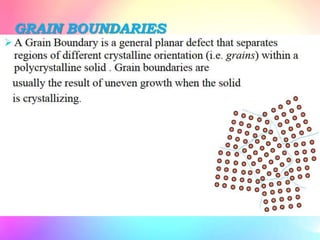
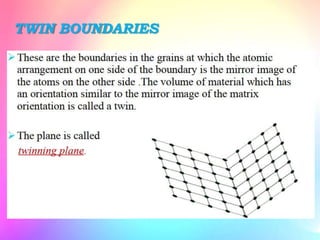
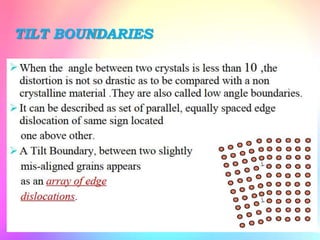
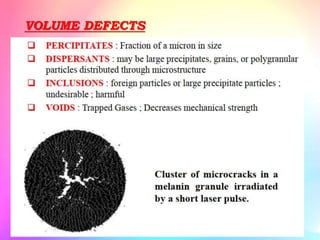
There are several types of crystal defects including point defects, line defects, surface defects, and volume defects. Point defects involve irregularities around a single atom and include vacancies, interstitials, and Frenkel and Schottky defects. Line defects are distortions along a line called dislocations including edge and screw dislocations. Surface defects occur on crystal surfaces and include grain boundaries, twin boundaries, and stacking faults. Volume defects involve larger clusters of missing atoms forming cracks, voids or non-crystalline inclusions. The presence of defects significantly impacts material properties like strength, ductility, and electrical conductivity.