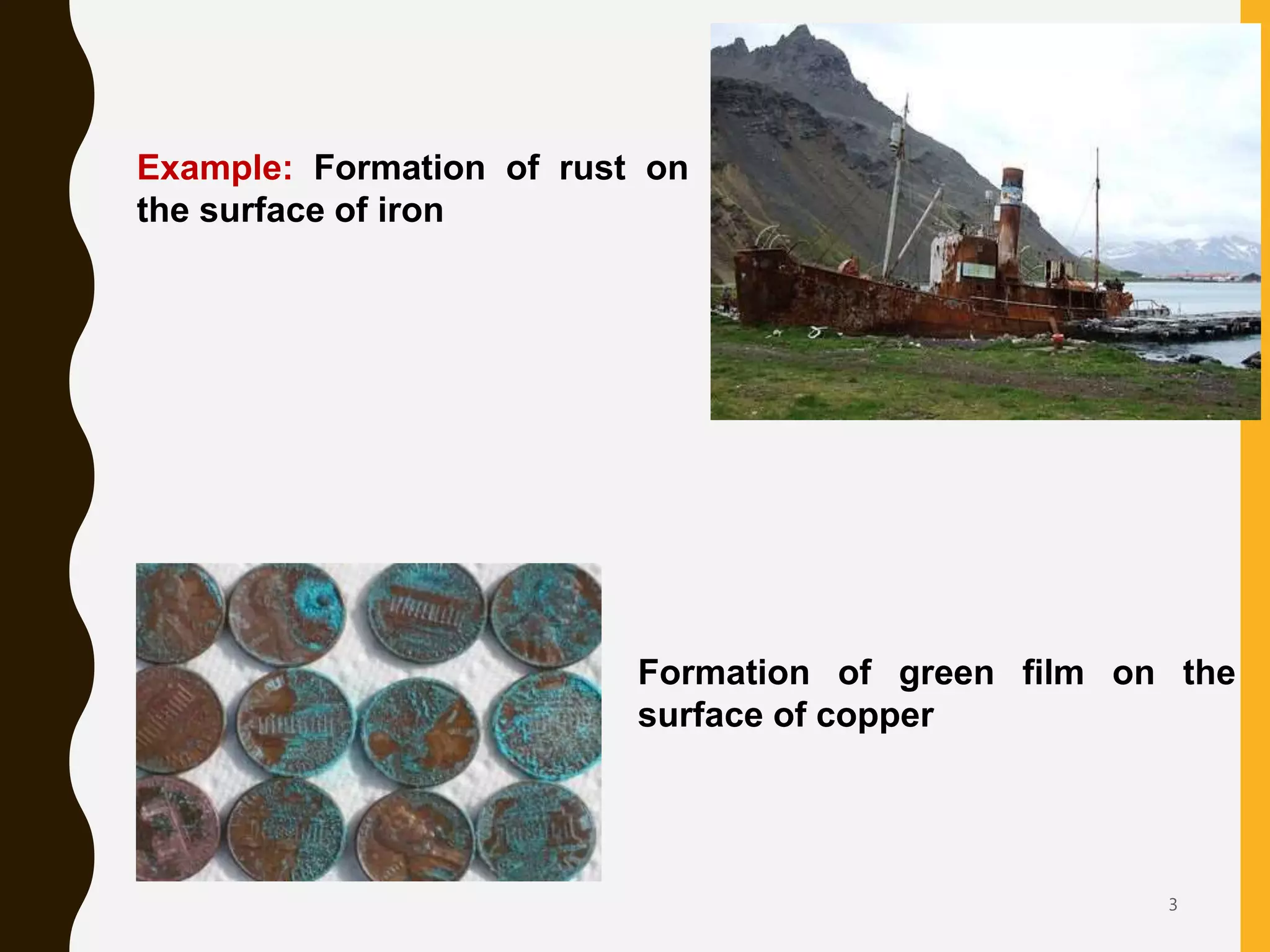
This document discusses various forms of corrosion that can occur in metals. It begins by defining corrosion and explaining the factors that influence it. It then describes several specific types of corrosion: general/uniform corrosion, galvanic corrosion, crevice corrosion, pitting corrosion, intergranular corrosion, dealloying, erosion corrosion, stress corrosion, hydrogen damage including hydrogen blistering and hydrogen embrittlement. For each type of corrosion, the document discusses the mechanism and provides methods for prevention.