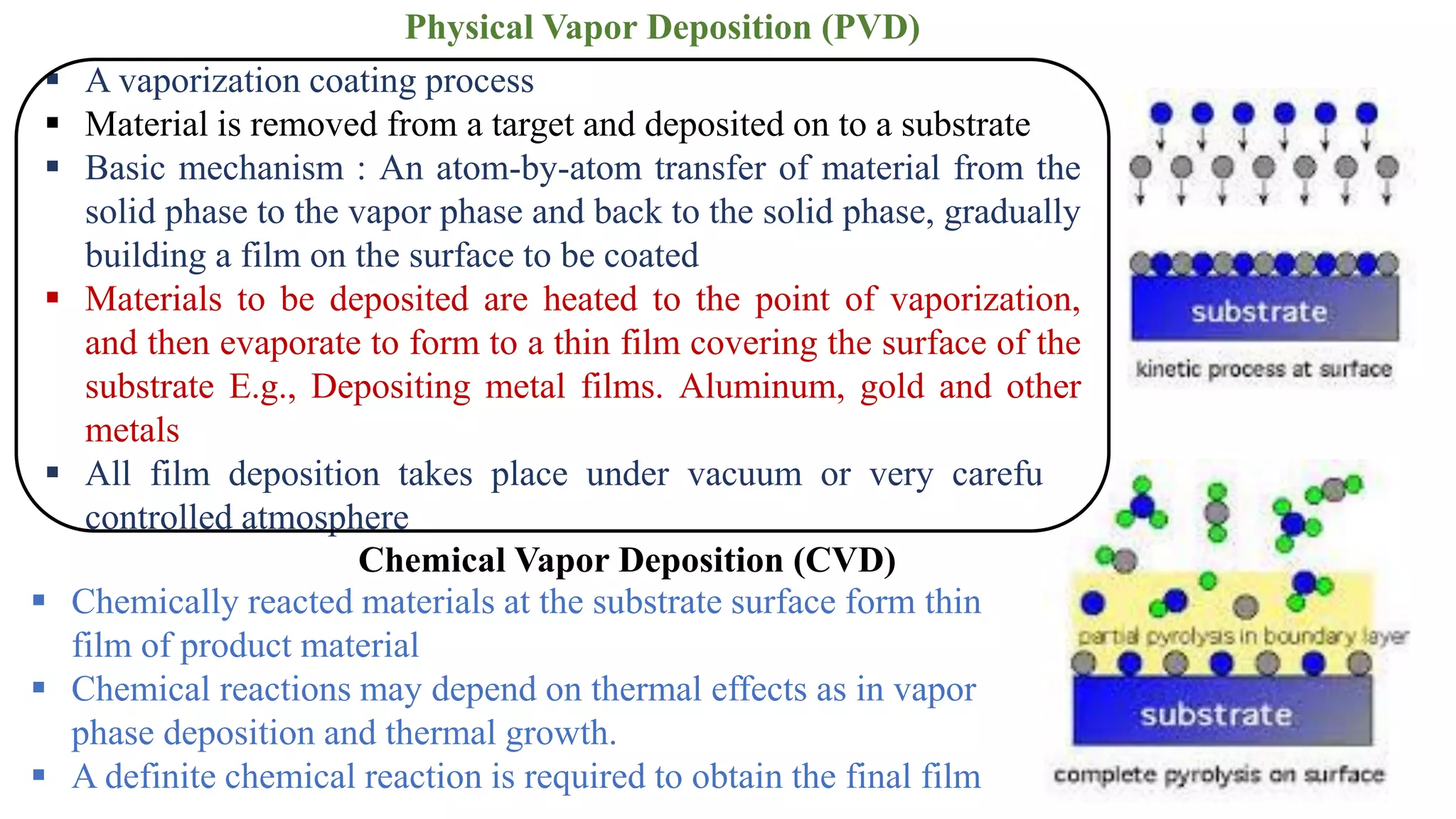
This document provides information on preparing thin films using the Successive Ionic Layer Adsorption and Reaction (SILAR) method. It discusses what thin films are, common thin film deposition techniques like physical vapor deposition and chemical vapor deposition, and the SILAR method specifically. SILAR involves alternating immersion of a substrate in cationic and anionic precursor solutions to deposit materials like cadmium sulfide in a layer-by-layer process. Parameters like concentration, pH, temperature, and deposition time must be optimized to produce adherent thin films. The document also outlines some applications of SILAR-deposited cadmium sulfide thin films and factors that influence thin film characteristics.







![Precursors: Cadmium chloride CdCl2 solution
Sodium sulphide Na2S solution
Substrate: Precleaned Glass or FTO
Deposition parameters for getting adherent
thin films
▪ Concentration of the solution
▪ pH of the reacting baths,
▪ temperature of deposition
▪ Substrate treatment
▪ Deposition time
Under optimized deposition conditions
only, adherent thin films can be produced
Preparation of Cadmium sulphide CdS thin films
• A chemical dipping technique
• Involves successive dipping of a precleaned
substrate in separately placed cationic and
anionic precursor
• Between every immersion it is rinsed in ion
exchanged water
Na2S
solution
CdCl2
⋅2H2O]
solution
Adsorption of
cationic metal ions
Washing
Adsorption of
anionic ions
Reaction on the substrate
lead to the formation of
metal sulphide ion
Washing](https://image.slidesharecdn.com/preparationofthinfilms-210910032820/75/Preparation-of-thin-films-9-2048.jpg)
![Cadmium sulphide -optoelectronic applications
▪ solar cells
▪ photodiodes
▪ light emitting diodes
▪ nonlinear optics
▪ heterogeneous photocatalysis
▪ high-density magnetic information storage
Precursors: Cadmium acetate Cd(CH3COO)2 ⋅2H2O] solution
Ammonium sulphide [(NH4)2S] solution (OR)
Sodium sulphide Na2S solution
Substrate: Precleaned Glass or FTO
[(NH4)2S]
solution
Cd(CH3CO
O)2 ⋅2H2O]
solution
Adsorption of
cationic metal ions
Washing
Adsorption
of
anionic ions
CdS formation on the
substrate
Preparation of Cadmium sulphide CdS thin films
Cd(NO2)2 Cationic solution
Sodium sulphide Na2S solution
Washing
▪ The characteristics of thin films are very much
dependent on their thickness as the thickness of the
film resolves the performance of prepared thin films
▪ Thin films surface roughness, contact angle, adhesion
and intrinsic stress are the factors which decide the
efficiency of the operation of any device.
Significance of film thickness](https://image.slidesharecdn.com/preparationofthinfilms-210910032820/75/Preparation-of-thin-films-10-2048.jpg)

