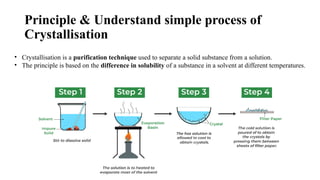
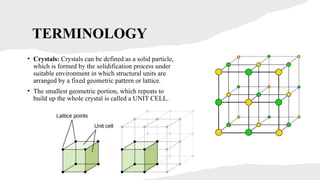
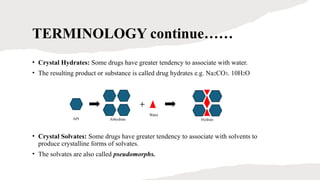
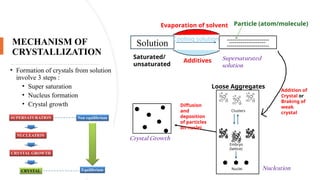
This presentation explores the process of crystallisation—a technique used to separate solids from a solution in pure form. It covers the basic principles, steps involved, types of crystallisation, and real-world applications in industries like pharmaceuticals, food processing, and chemical manufacturing. Ideal for students, educators, and professionals looking to understand or present this essential scientific method.