Embed presentation
Downloaded 1,440 times

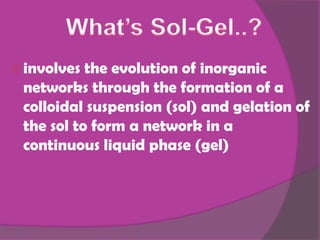

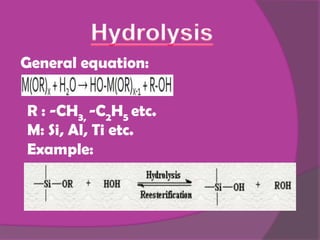
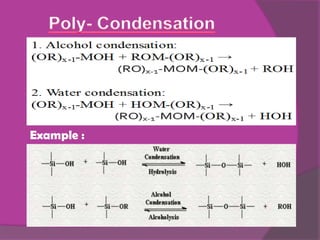



The sol-gel method involves creating an inorganic network through the formation and gelation of a colloidal suspension. Metal alkoxides and chlorides react with water through hydrolysis and polycondensation reactions to form this network. The sol-gel process is used to create protective coatings, thin films, fibers, and nano-scale powders for opto-mechanical applications and offers advantages over conventional glass production like low temperature operation and better control over material properties at the nano-scale.






