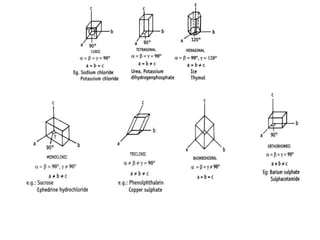
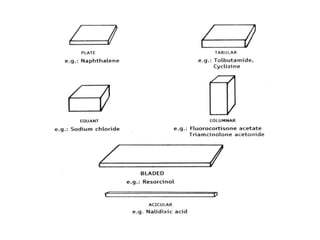
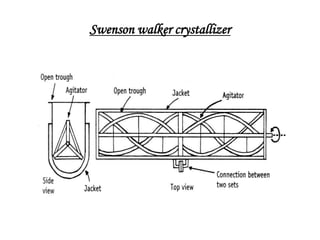
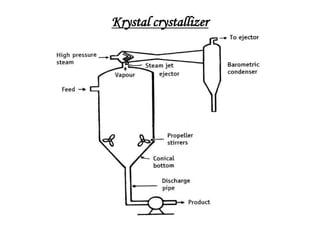
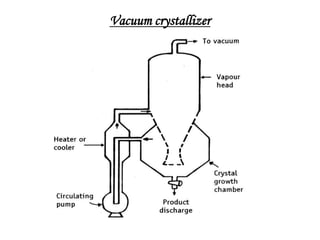
This document discusses the process of crystallization. It begins with an introduction that defines crystallization as the spontaneous arrangement of particles into repetitive, orderly arrays. It then describes three key characteristics of crystals: crystal lattice, crystal systems, and crystal habit.
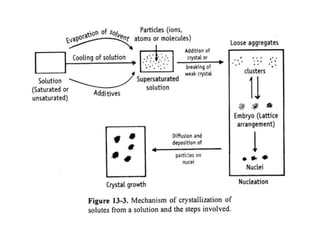
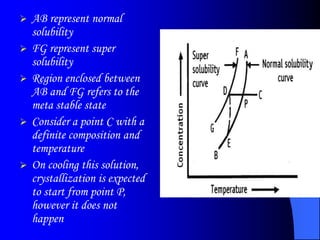
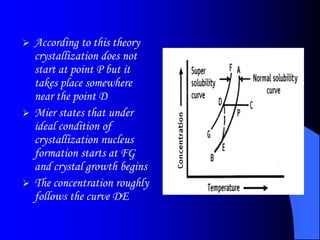
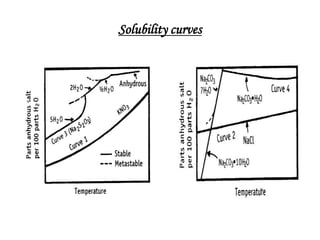
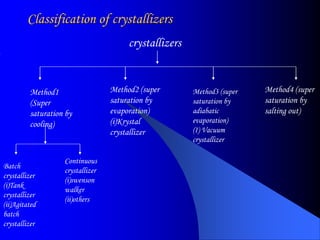
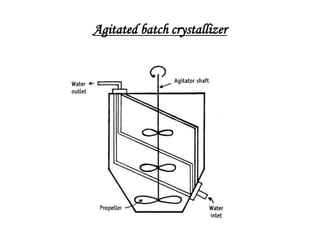
The bulk of the document discusses various aspects of crystallization theory and mechanisms. It explains super saturation, nucleation, and crystal growth. It also covers Miers' super saturation theory, solubility curves, types of crystallizers like batch and continuous crystallizers, and factors that influence caking of crystals. Numerical examples are provided to demonstrate calculating crystallization yield. The document concludes by listing references used.