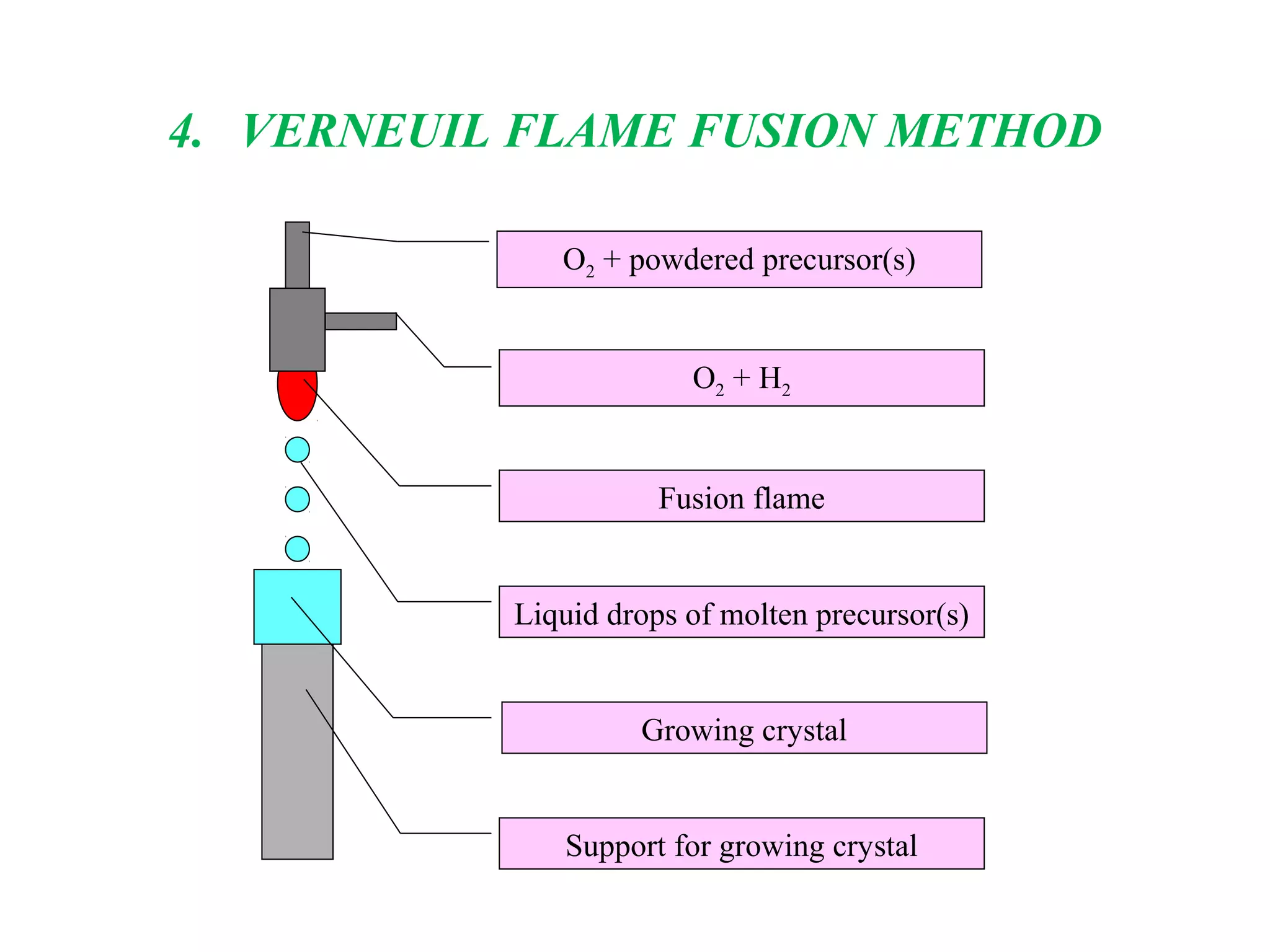
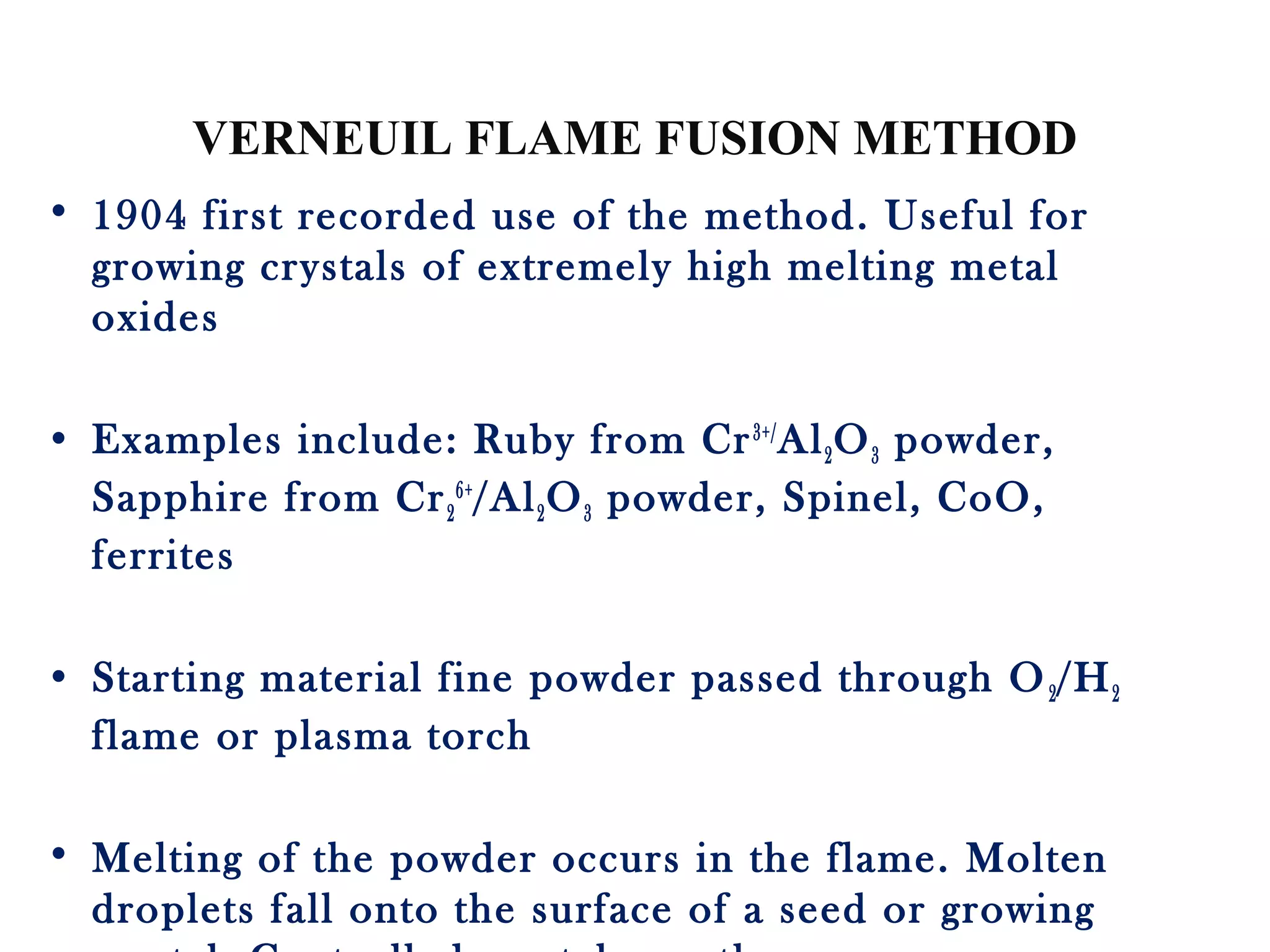
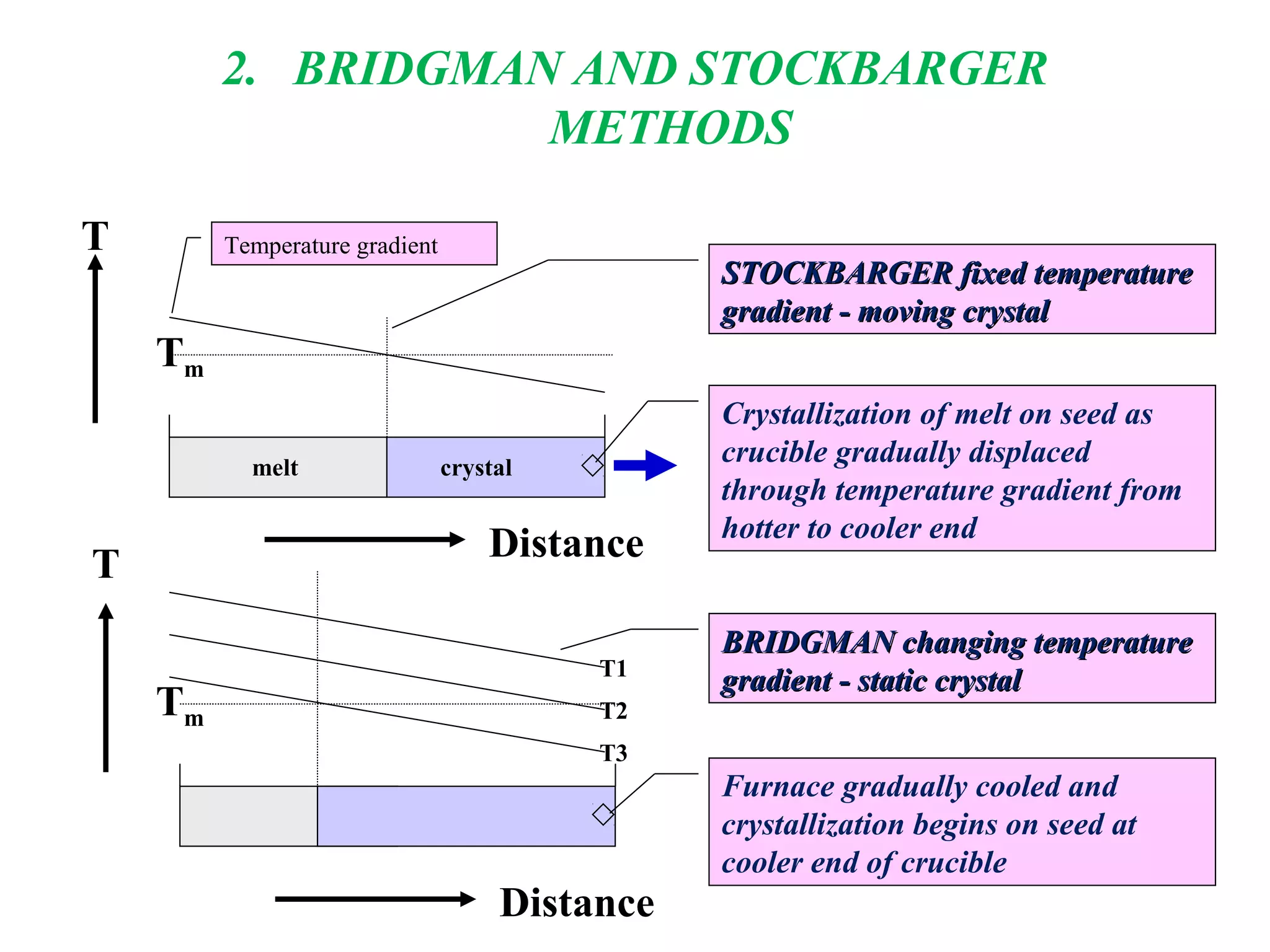
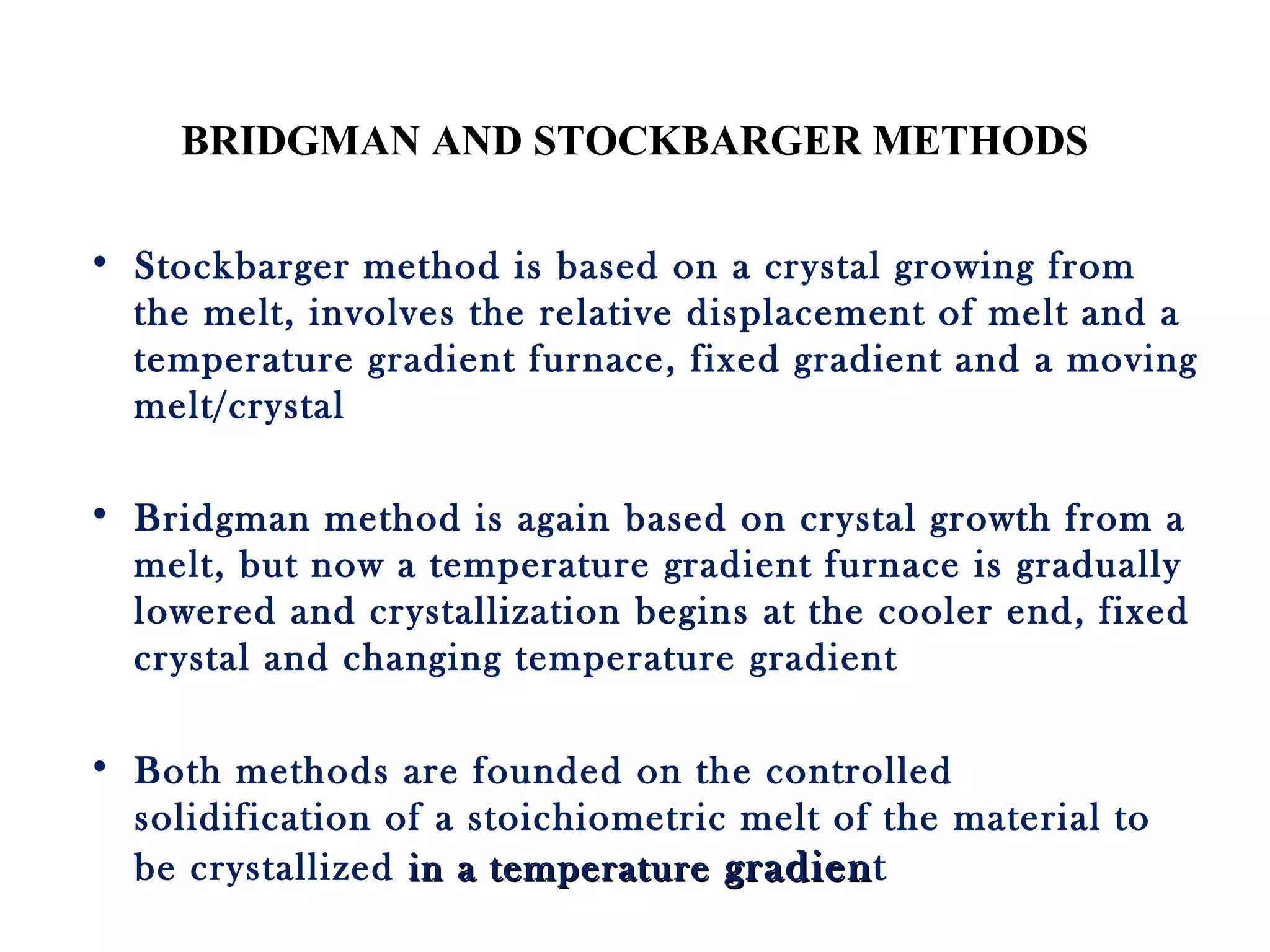
The document discusses several techniques for growing single crystals, which are important for measuring anisotropic properties and fabricating devices. The Czochralski technique involves pulling a crystal seed from a melt held just above its melting point to form a single crystal. The Bridgman and Stockbarger techniques use controlled solidification of a melt within a temperature gradient furnace. Zone melting involves melting a small region of a sample to purify it as impurities concentrate in the liquid. The Verneuil technique grows crystals by melting and solidifying powder precursors in an oxygen-hydrogen flame.
![GROWTH OF SINGLE CRYSTALSevices
[Paper I – Solid State Chemistry]
- Jaiswal Priyanka
M.Sc. II [Inorganic]
Mithibai College](https://image.slidesharecdn.com/growthofsinglecrystals-170615160554/75/Growth-of-single-crystals-1-2048.jpg)






![T
Distance
Crystal or powder
Localized melt region - impurities
concentrated in melt – energetic benefit
Crystal growing from seed
Temperature profile furnace
Pulling direction
Tm
3. ZONE MELTING
[CRYSTAL GROWTH AND PURIFICATION OF SOLIDS]](https://image.slidesharecdn.com/growthofsinglecrystals-170615160554/75/Growth-of-single-crystals-10-2048.jpg)