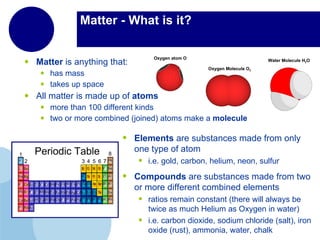
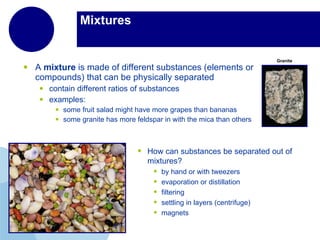
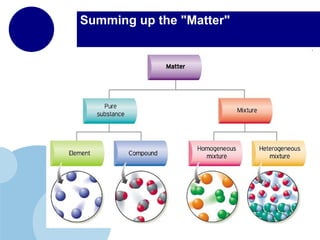
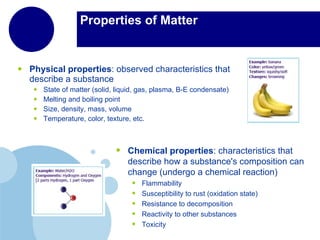
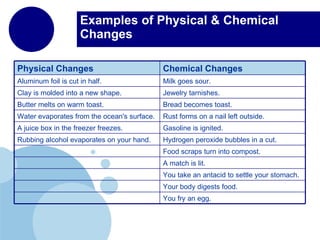
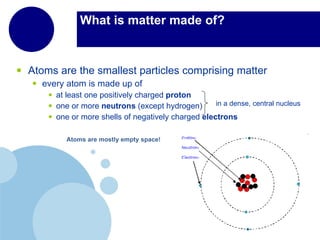
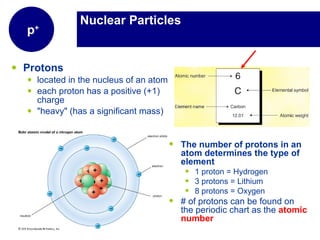
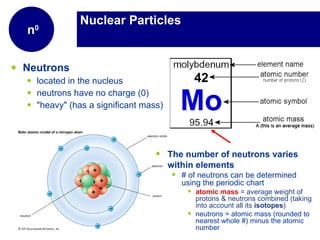
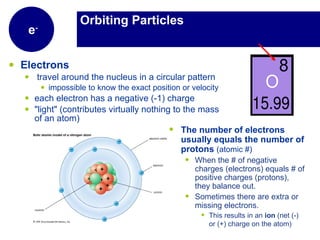
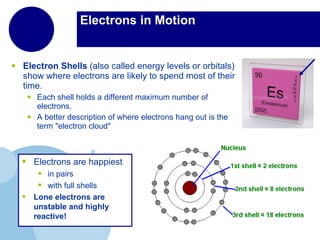
Matter is anything that has mass and takes up space. It is composed of atoms, which contain protons, neutrons, and electrons. Elements are pure substances made of only one type of atom, while compounds are made of two or more elements chemically bonded together. Mixtures contain different substances mixed together but not chemically combined. The three main states of matter are solids, liquids, and gases. Chemical and physical properties can be used to describe and identify matter.