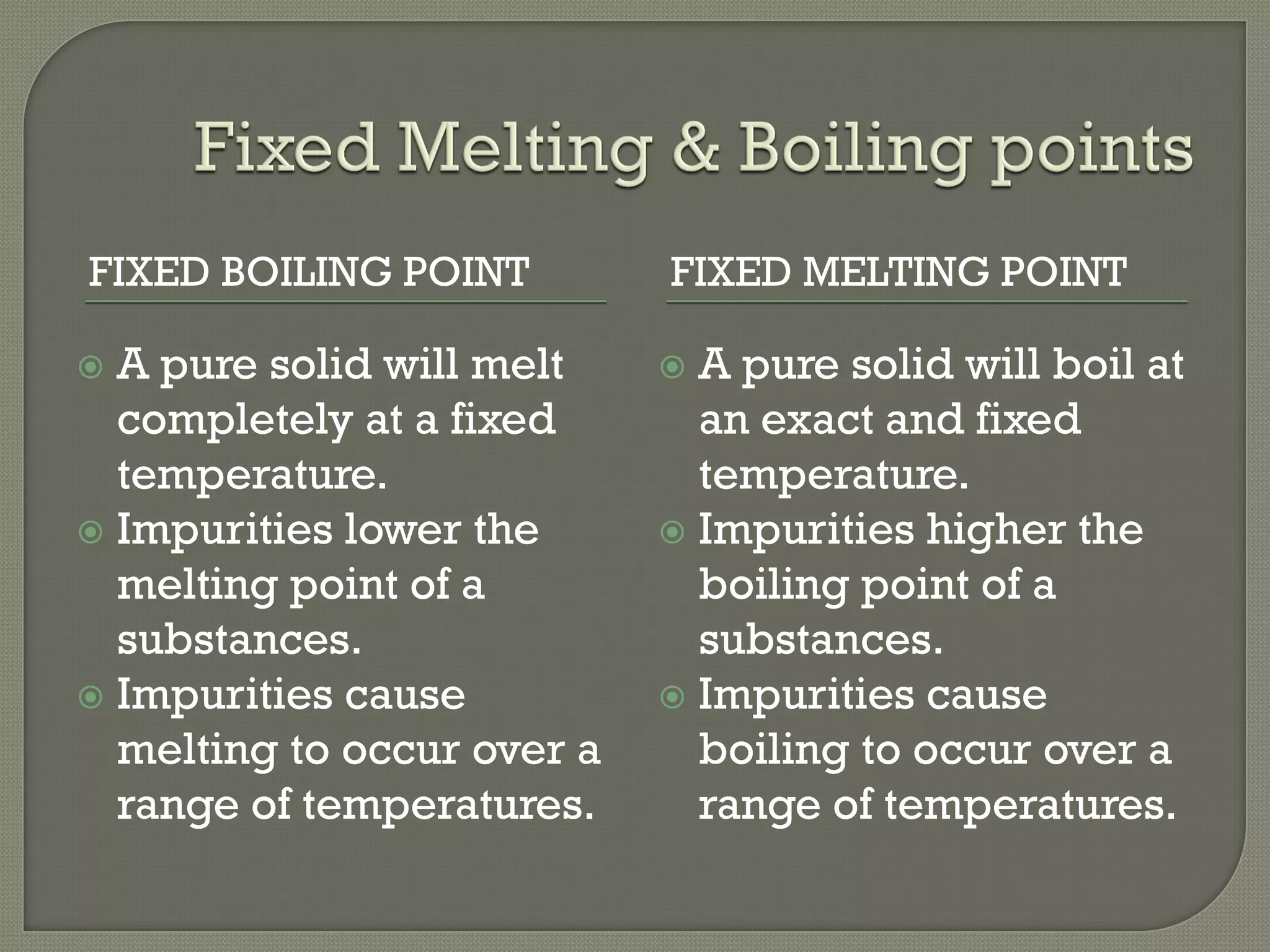
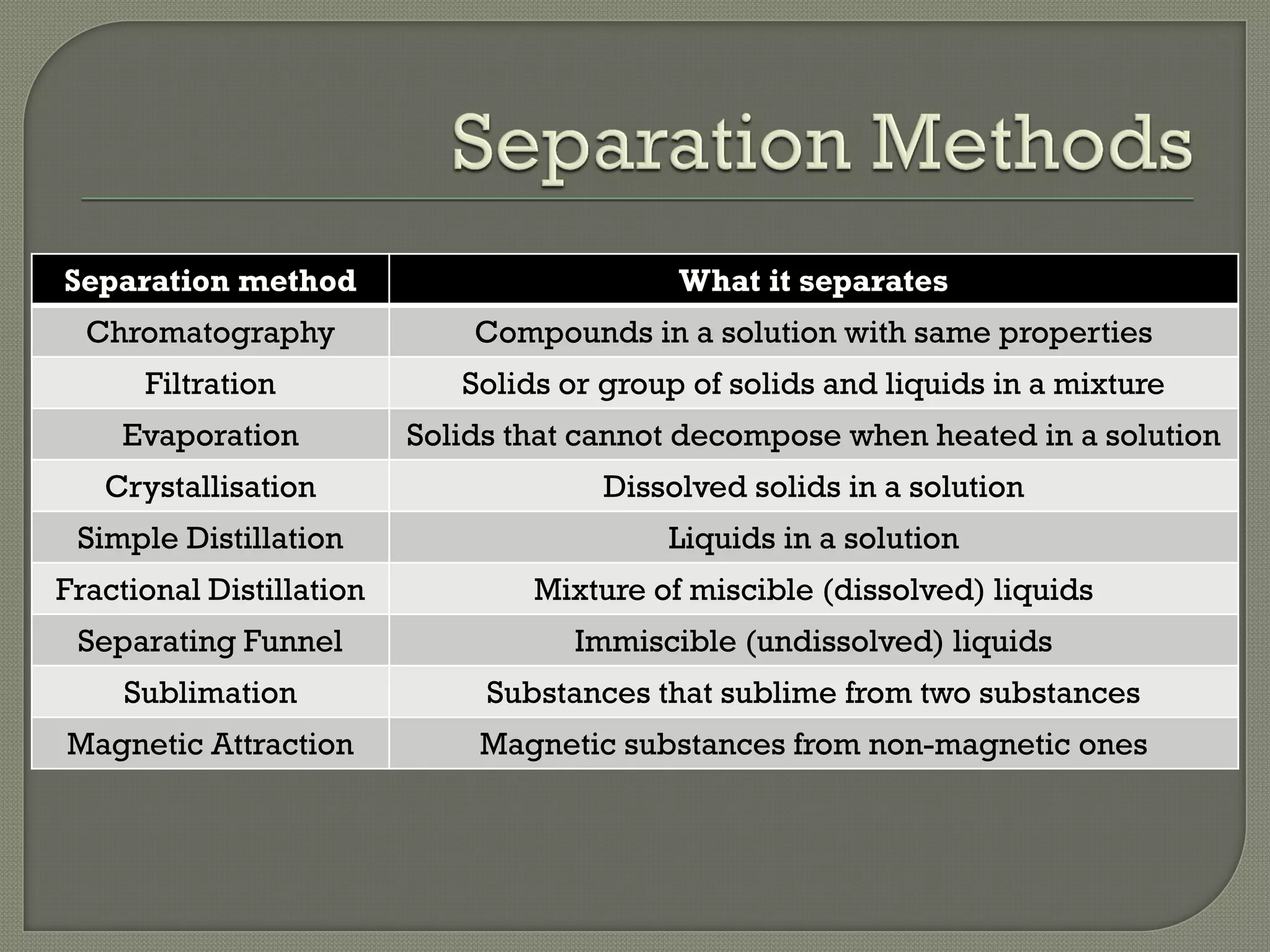
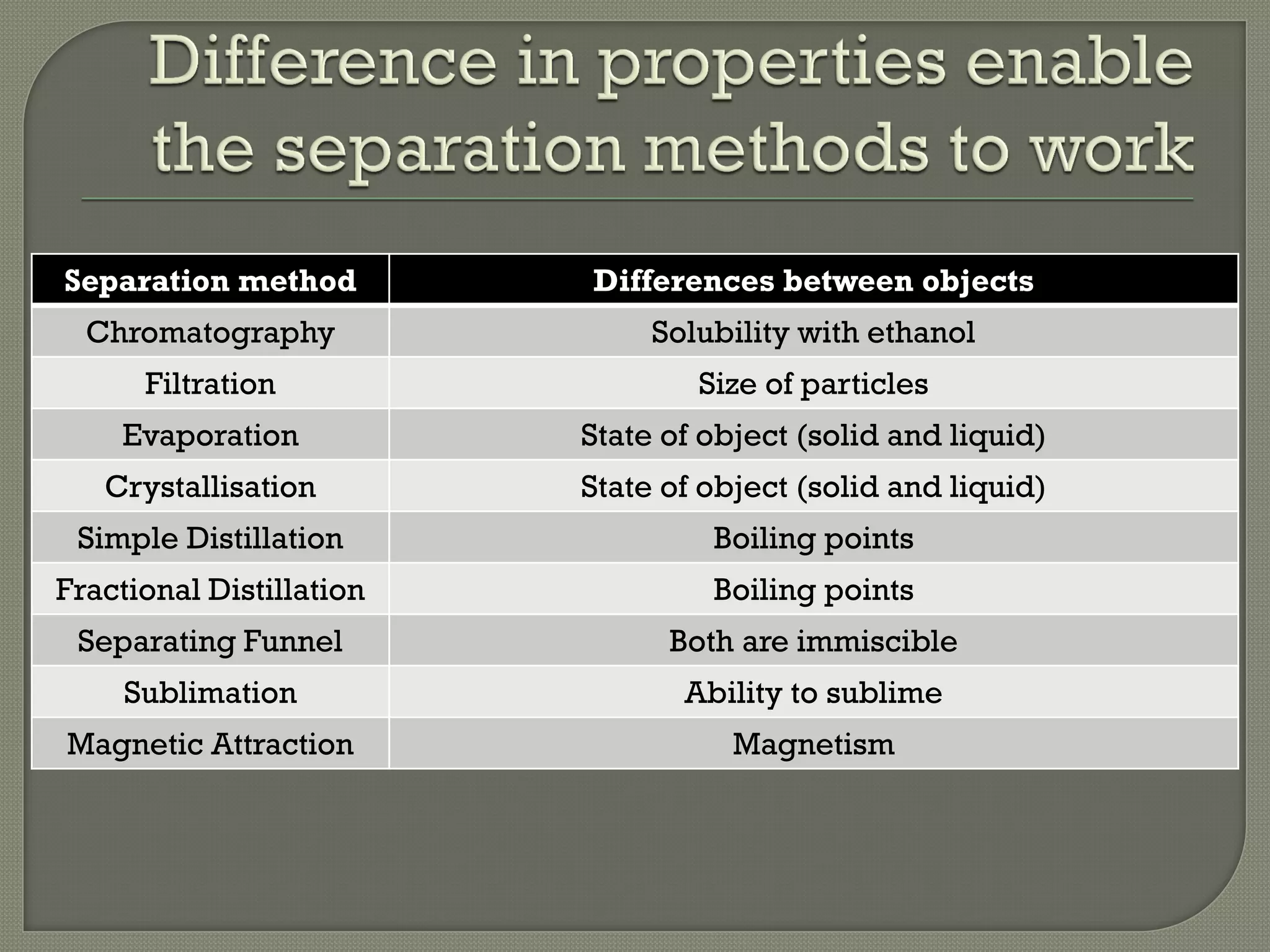
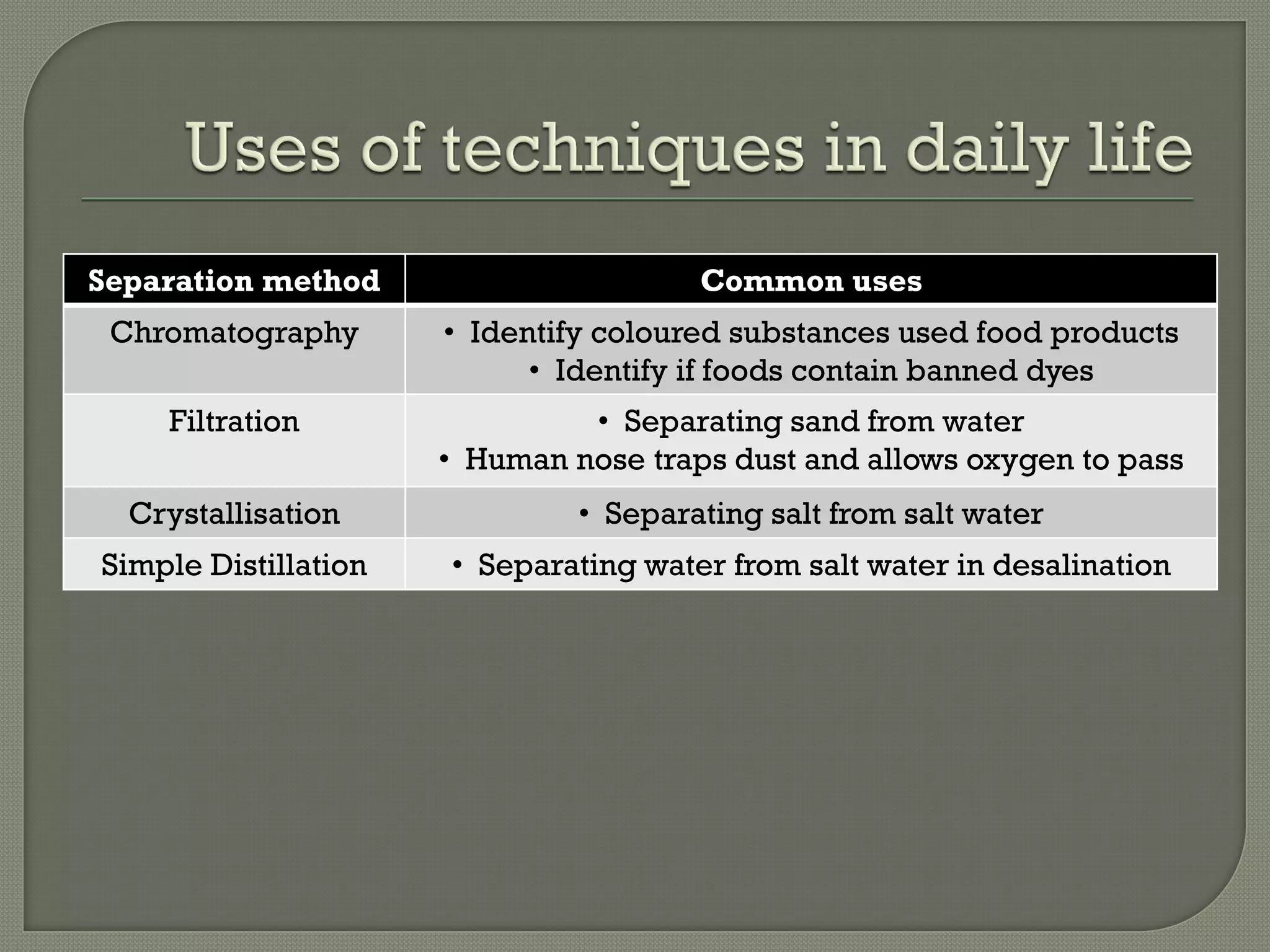
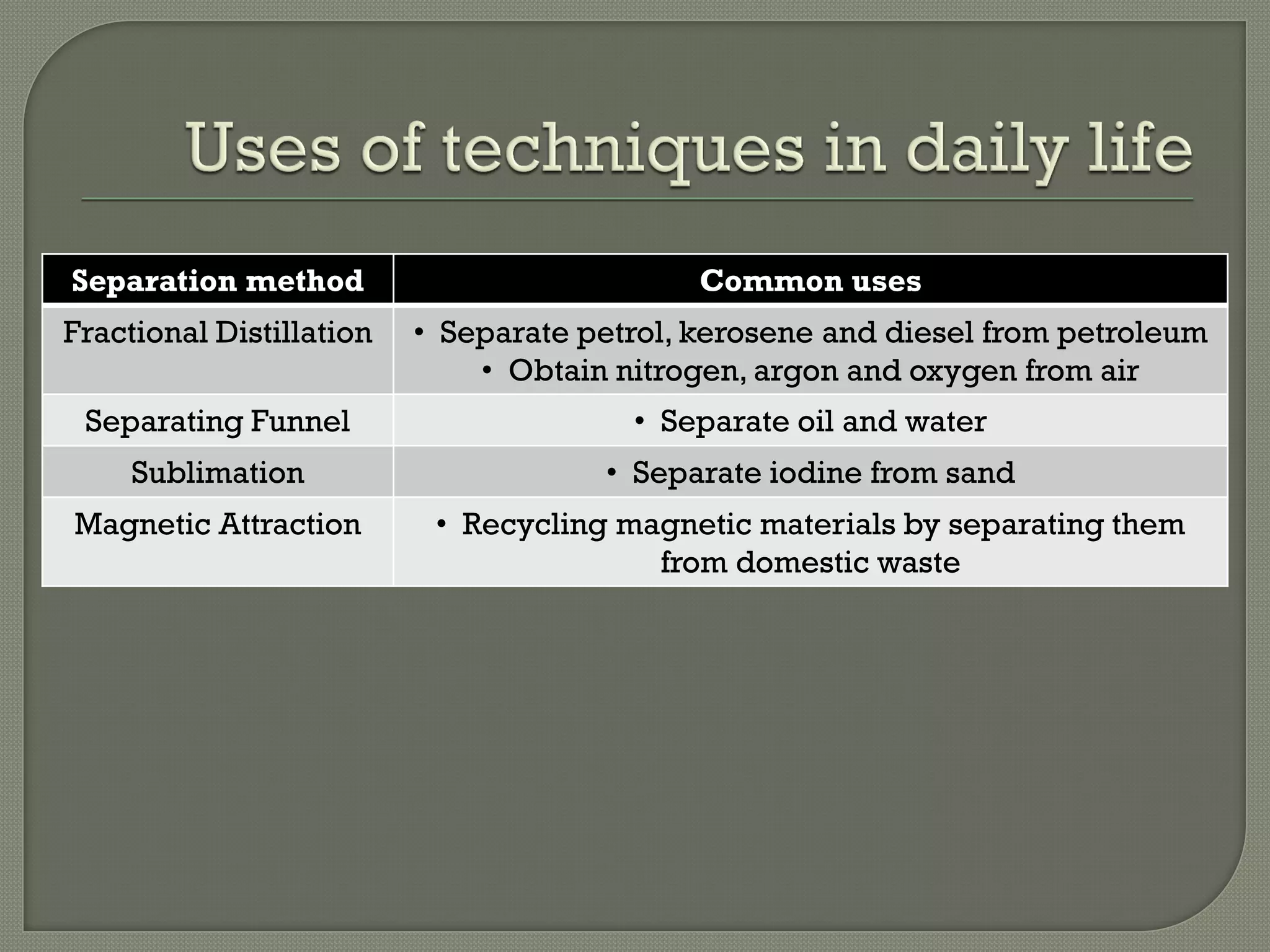
A pure substance consists of only one substance, while a mixture contains two or more substances not chemically combined. The purity of a substance can be determined by testing its fixed melting and boiling points or using chromatography. Various separation methods exist that separate substances based on differences in their physical properties such as solubility, boiling points, magnetism, and ability to sublime. Common separation methods include filtration, crystallization, distillation, chromatography, and magnetic attraction.