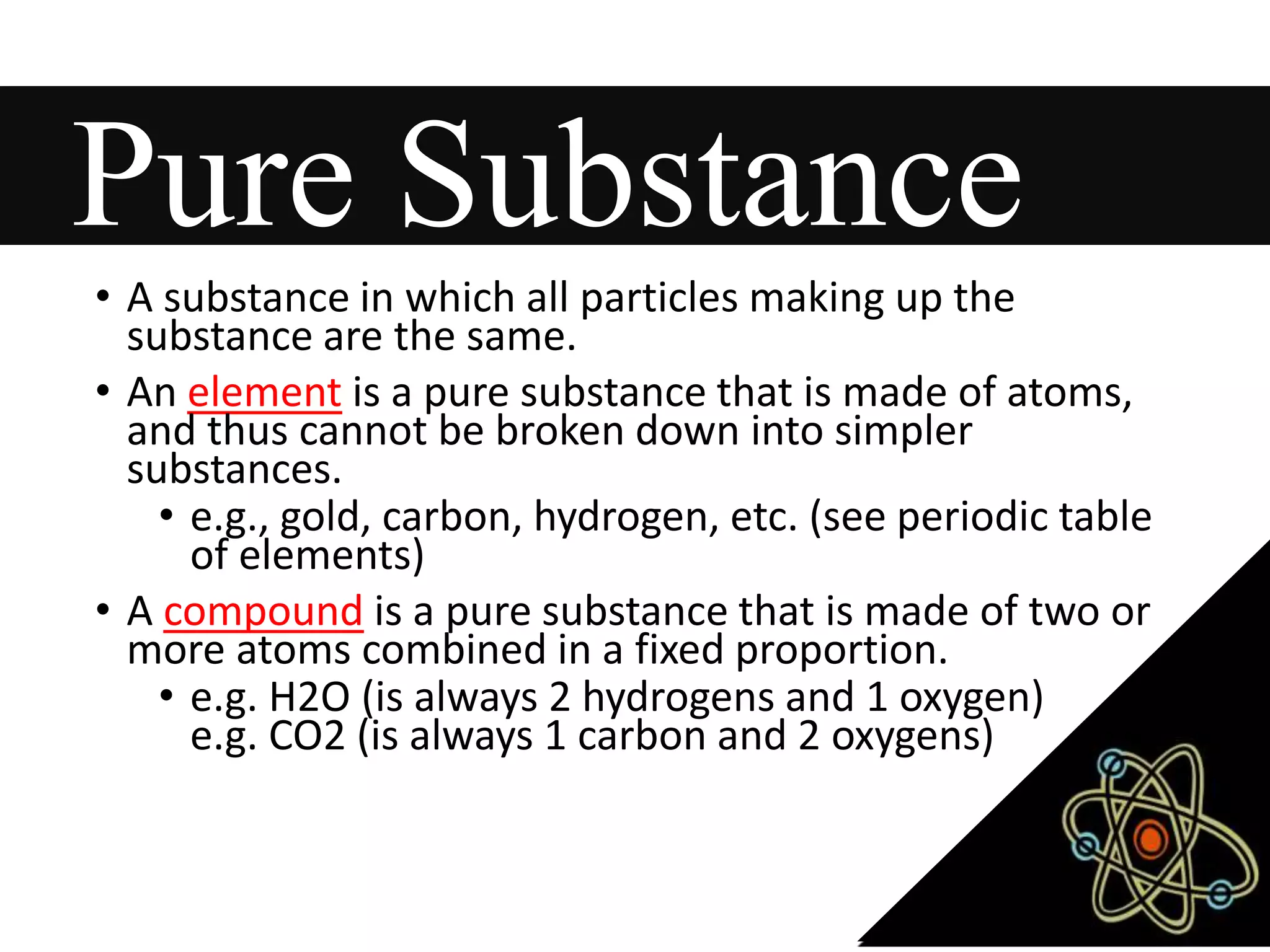
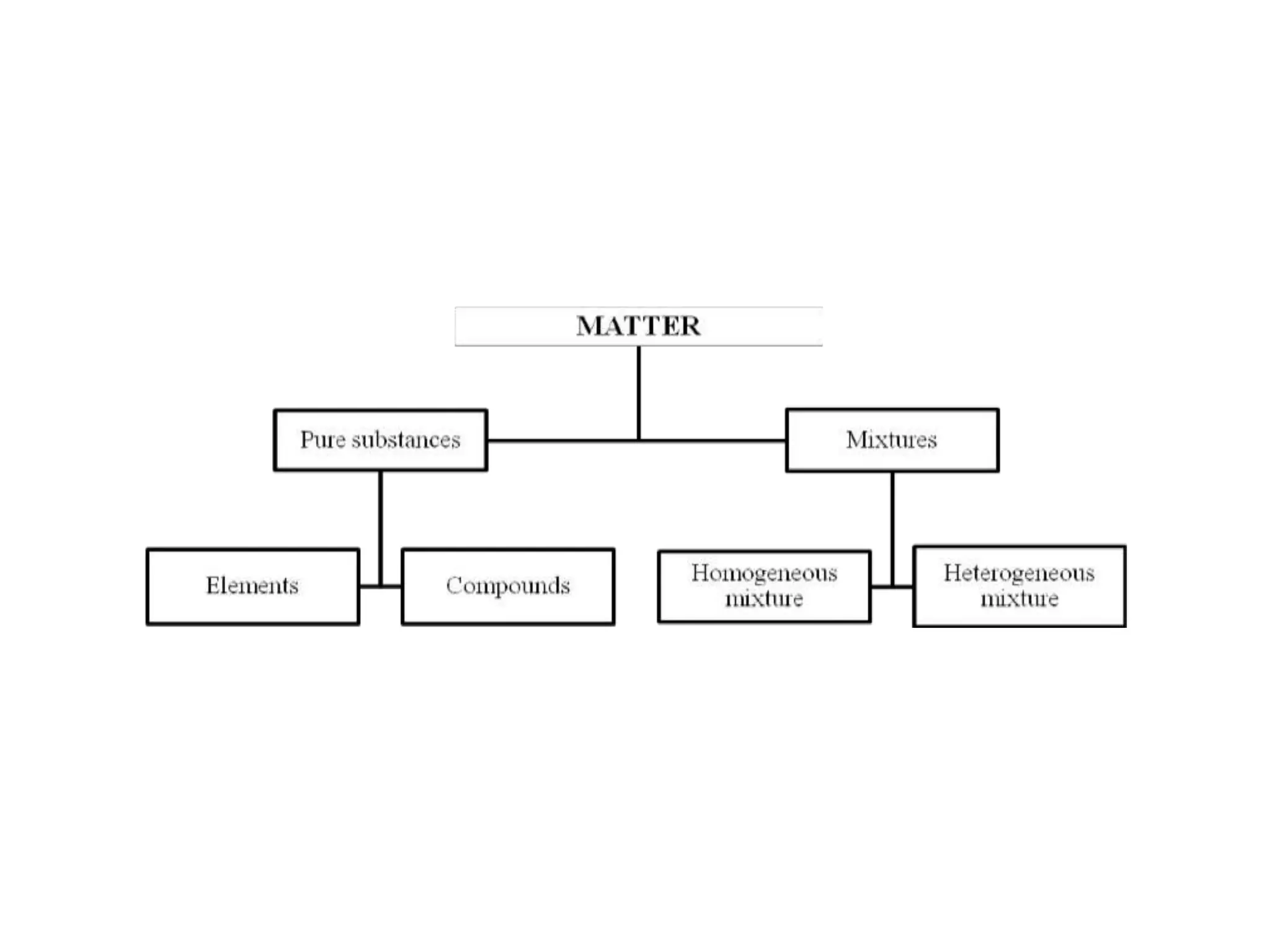
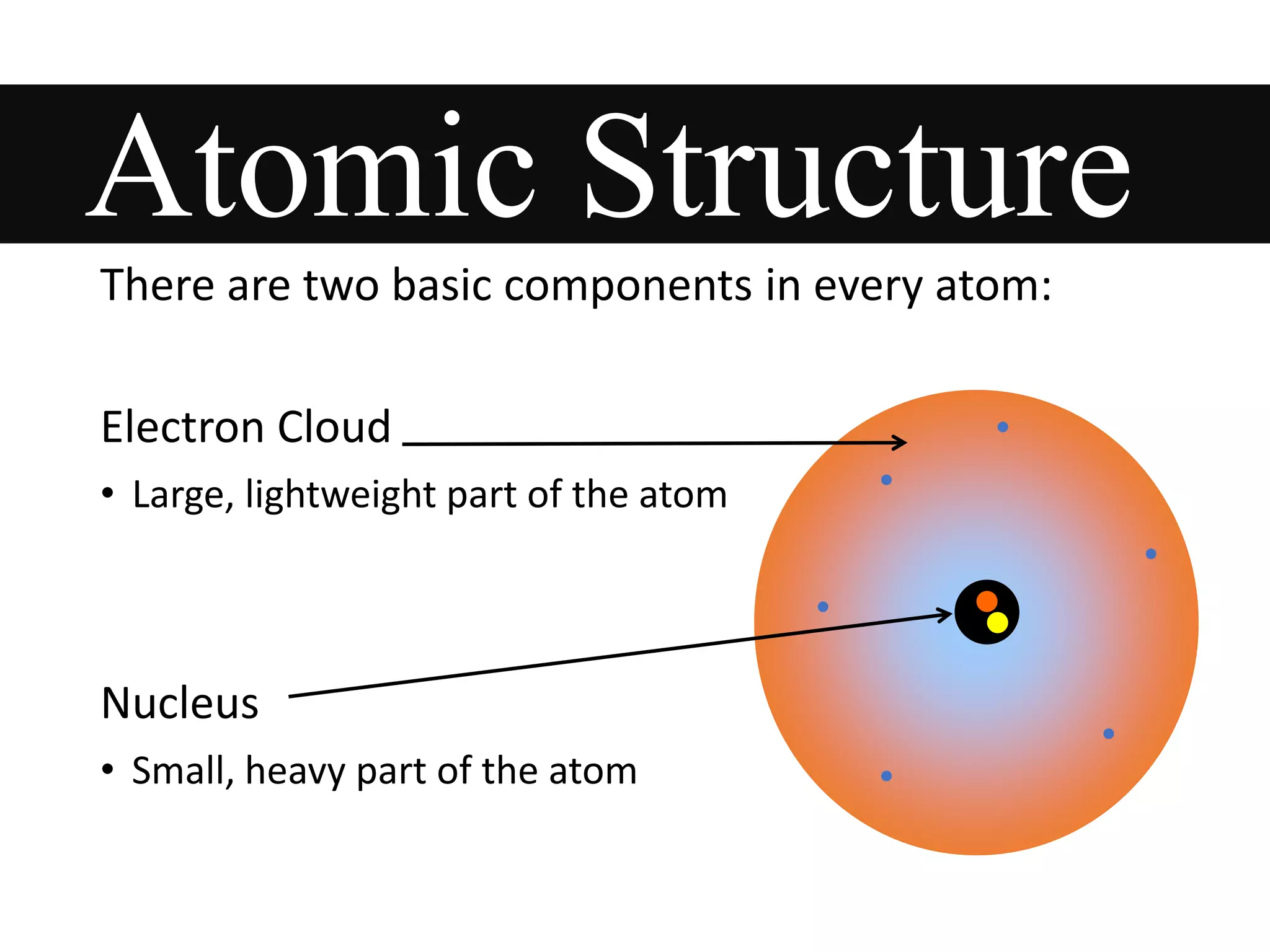
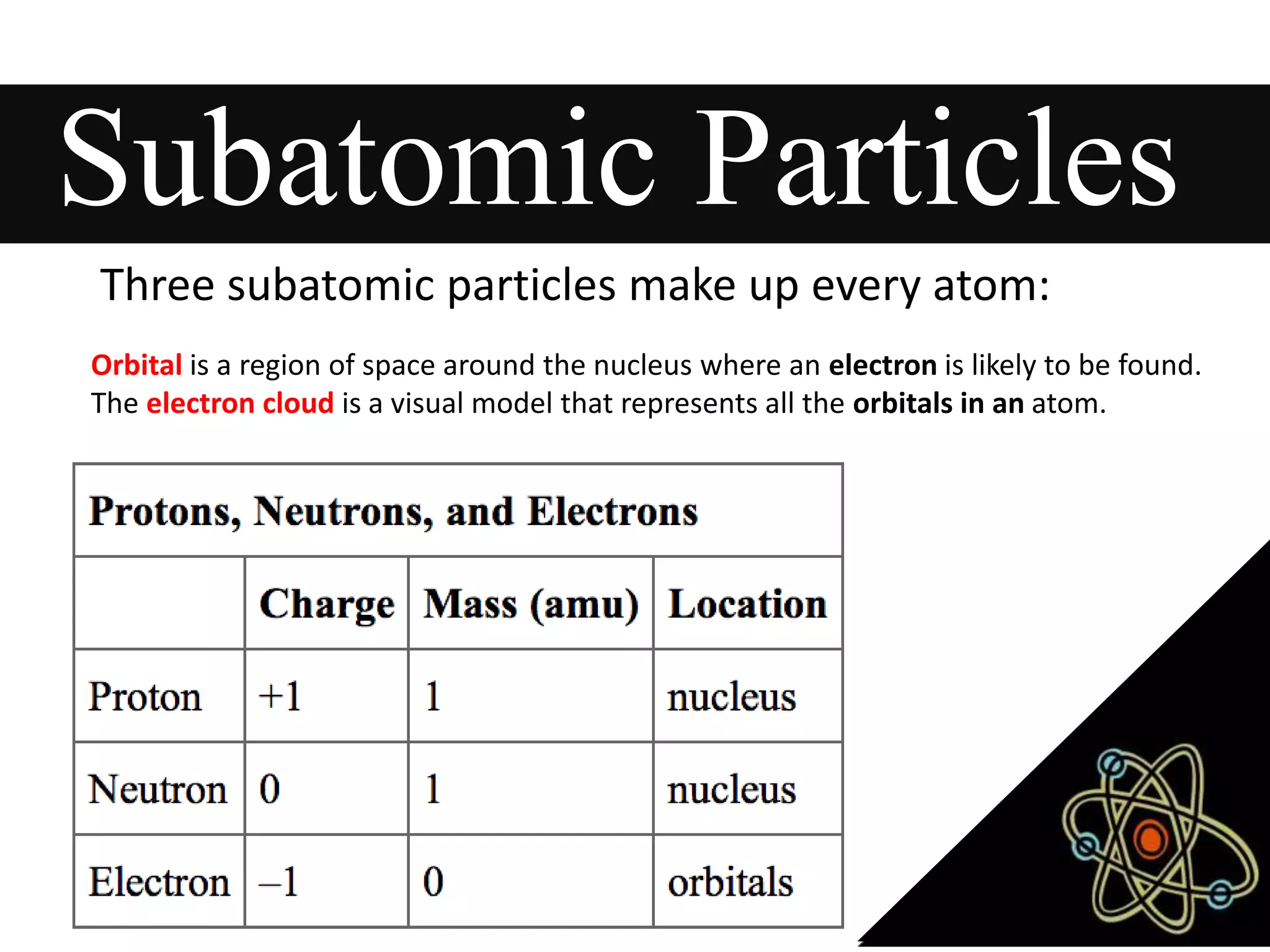
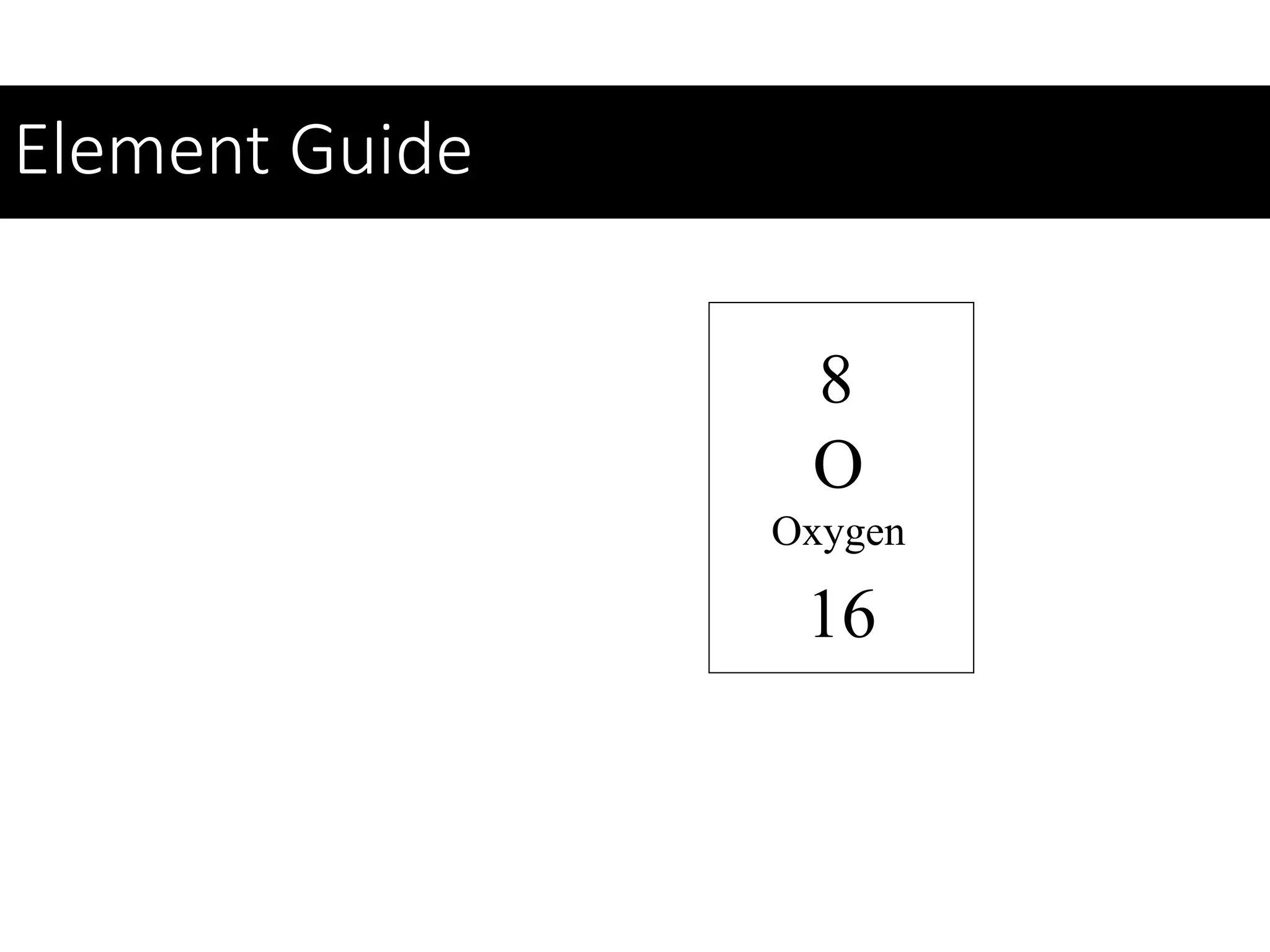
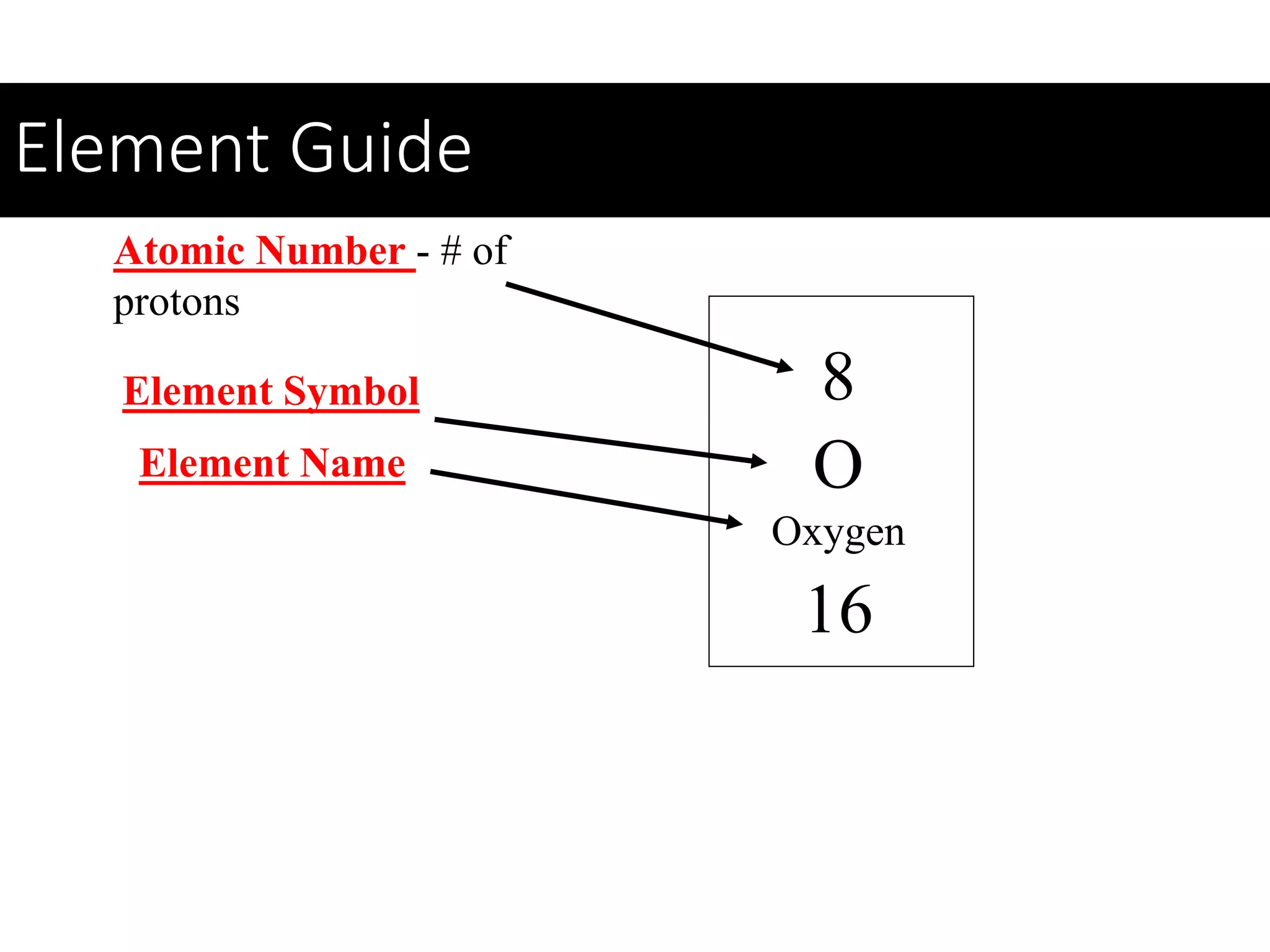
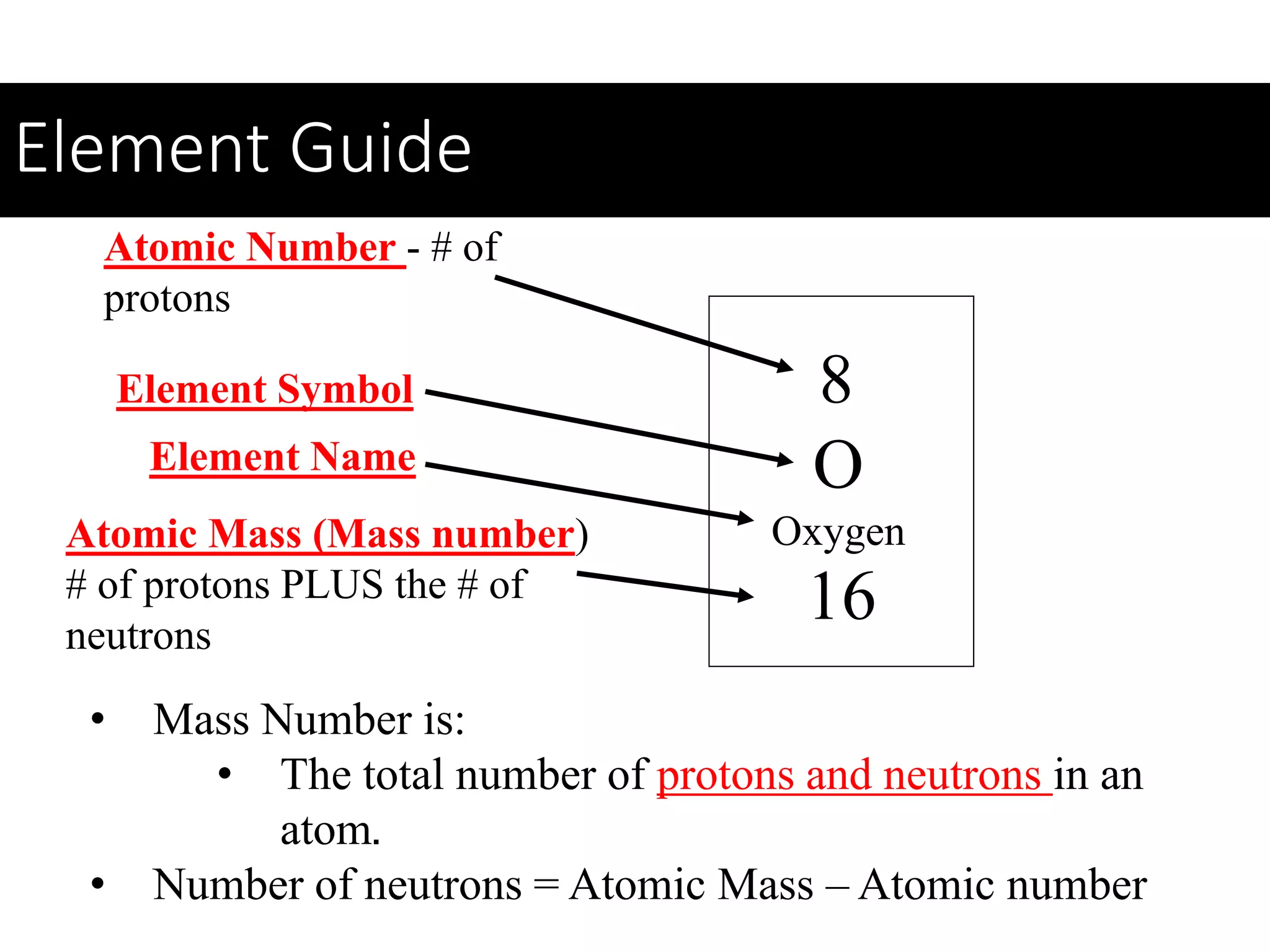
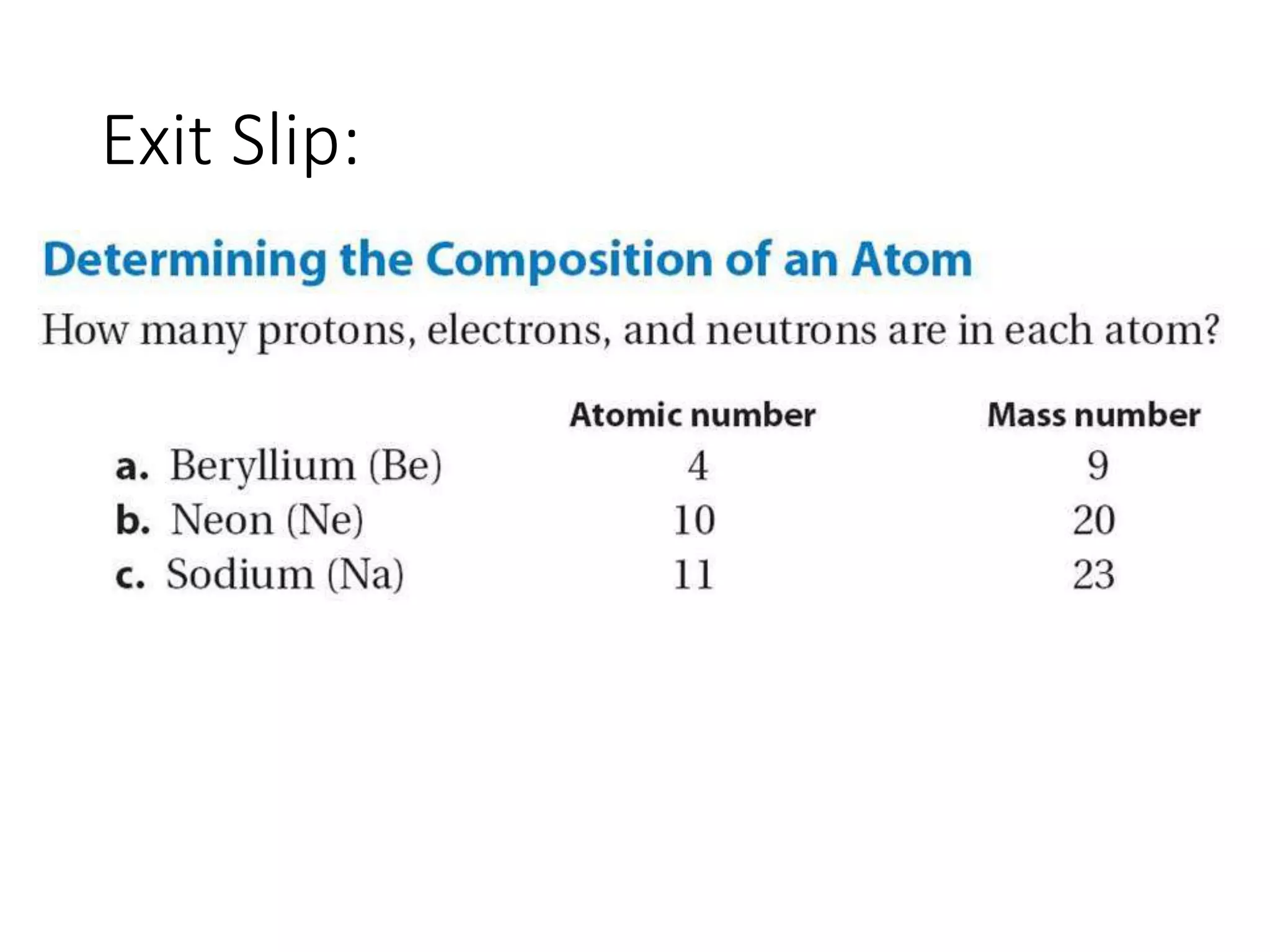
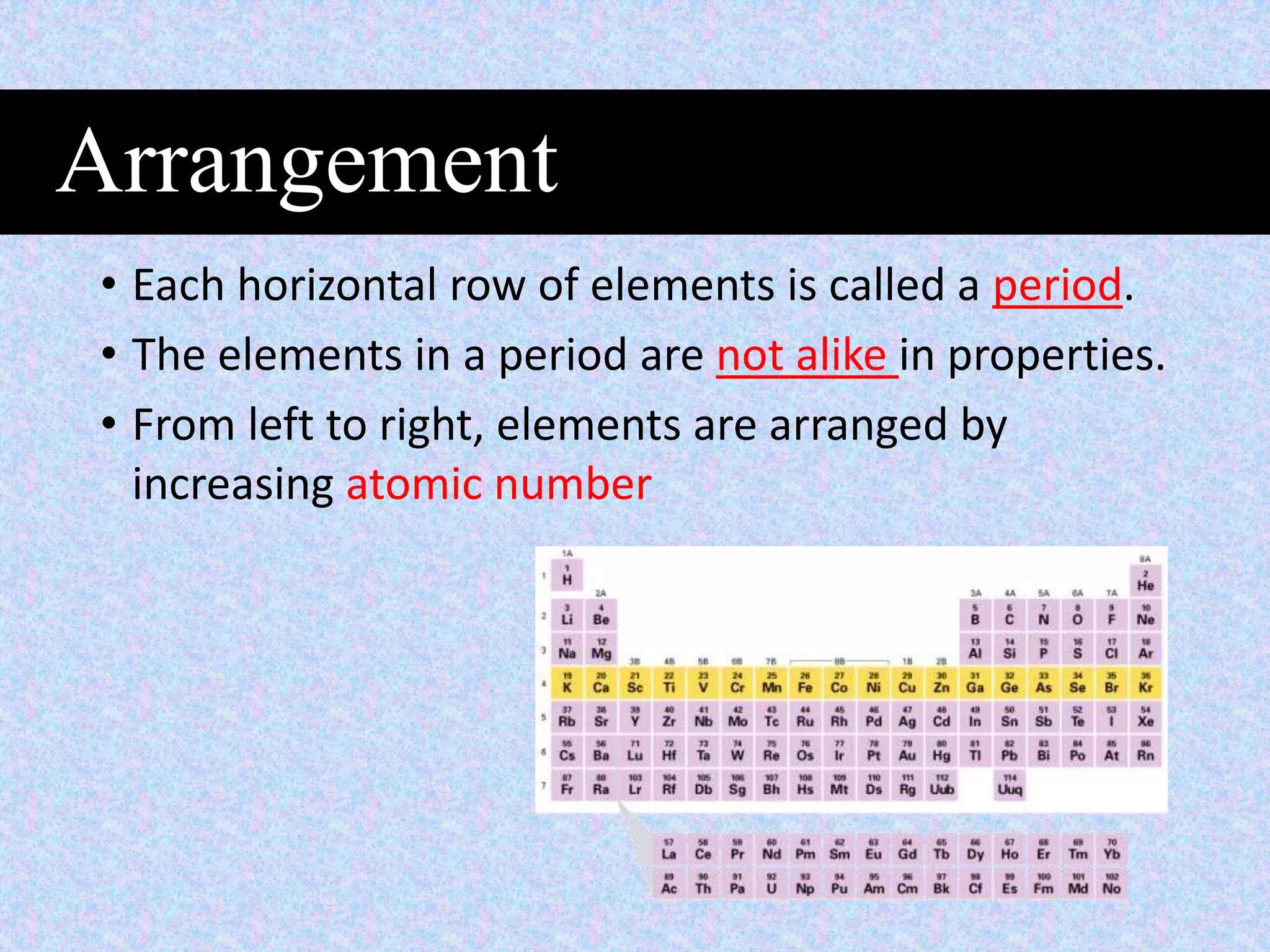
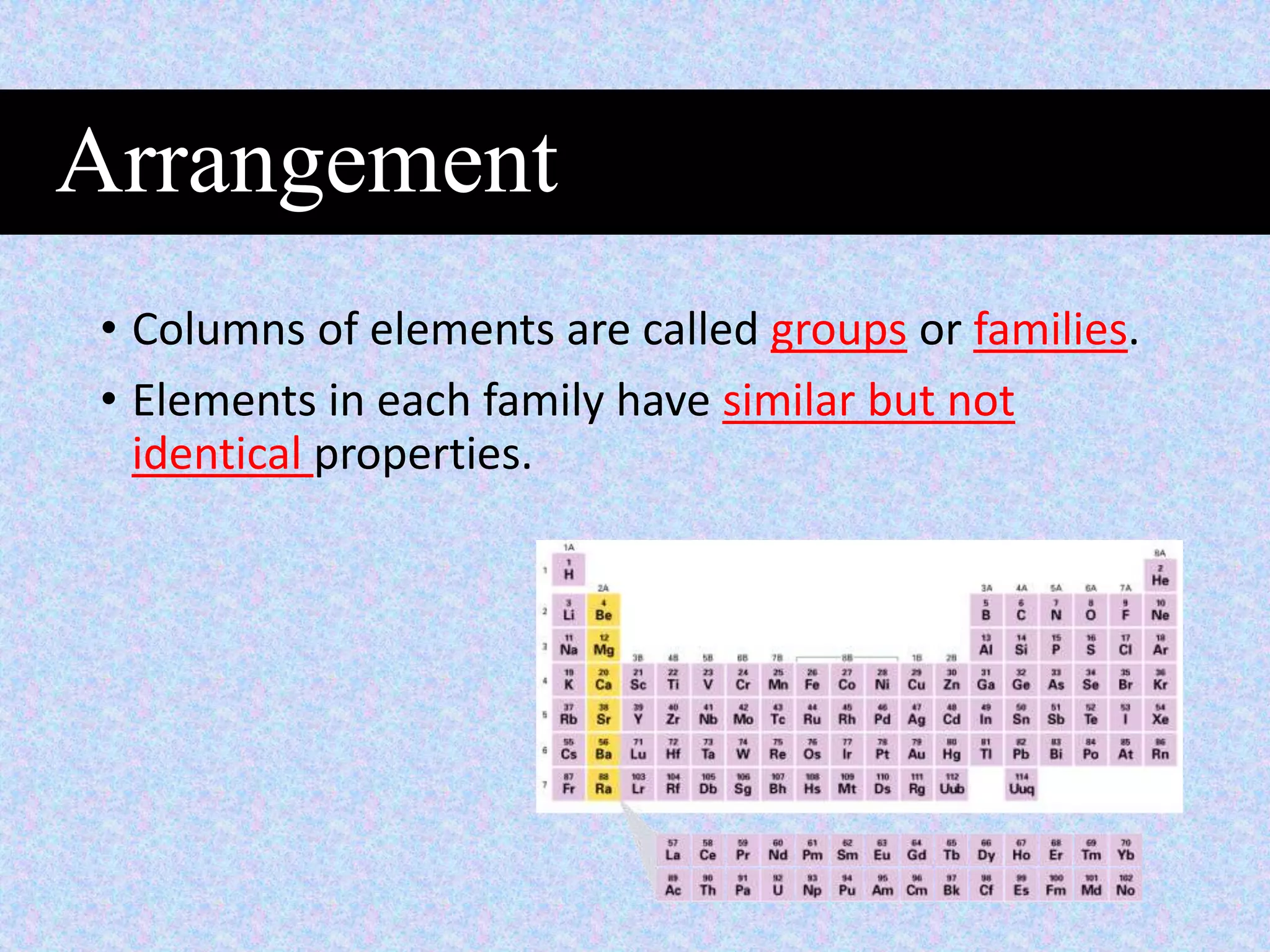
This document provides information about classifying matter and its composition. It defines pure substances as elements or compounds made of uniform particles and mixtures as substances with two or more types of particles. Pure substances undergo physical or chemical changes, which respectively involve changes in properties or the formation of new substances. The document also discusses atoms as the basic building blocks of matter, containing subatomic particles like protons, neutrons, and electrons. It introduces the periodic table as organizing the elements by their chemical properties and number of protons.