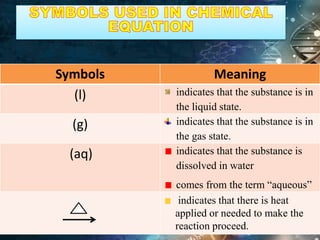
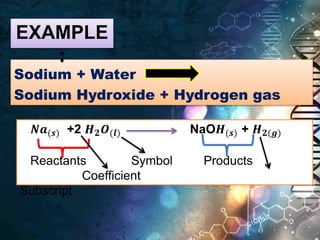
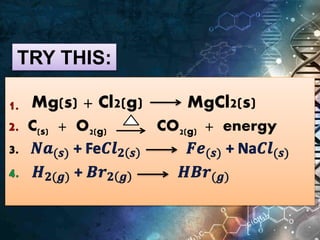The document defines key terms related to chemical equations:
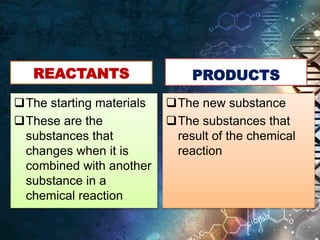
- Chemical reactions represent changes where reactants are converted to products through chemical changes.
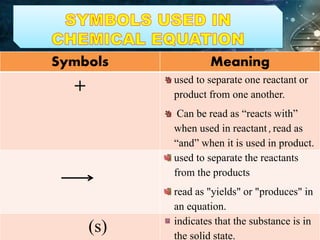
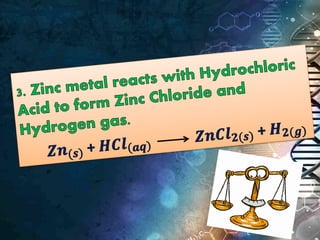
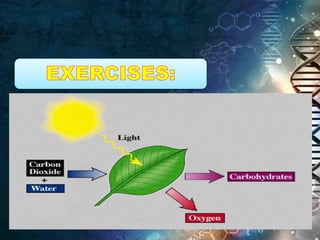
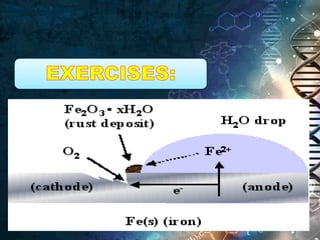
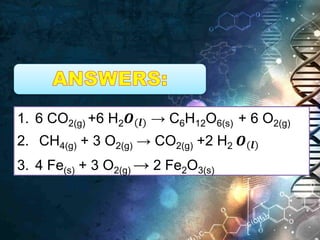
- Chemical equations express reactions using formulas, numbers, and symbols to represent reactants and products.
- Coefficients indicate the number of atoms or molecular units of each substance involved in the reaction.