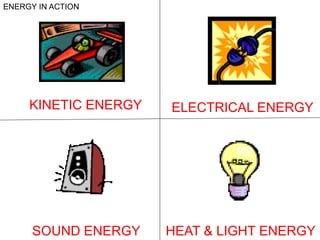
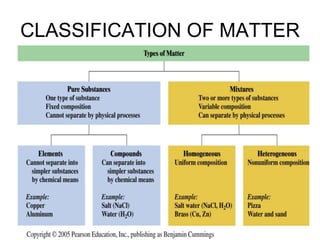
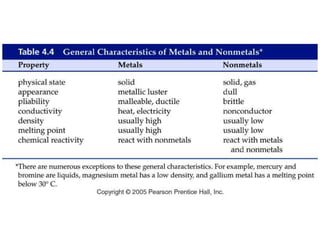
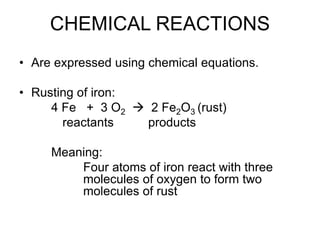
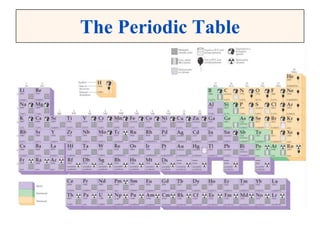
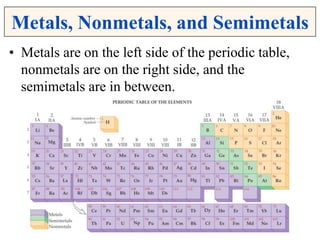
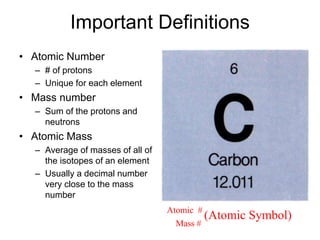
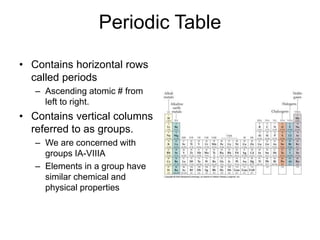
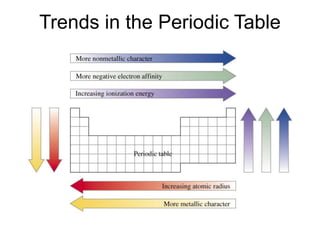
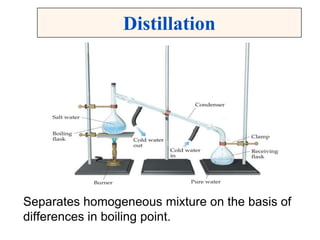
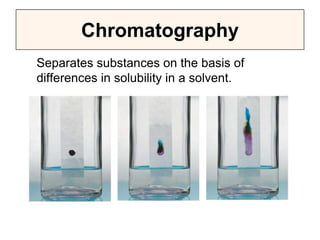
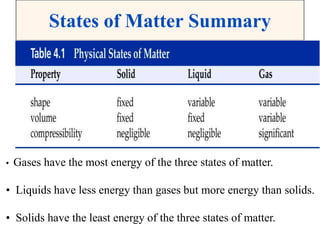
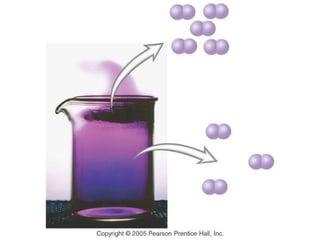
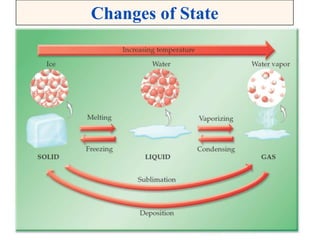
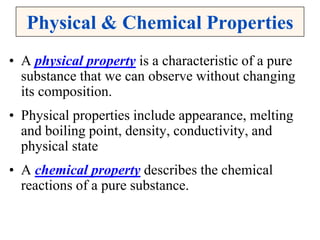
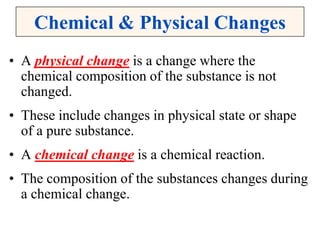
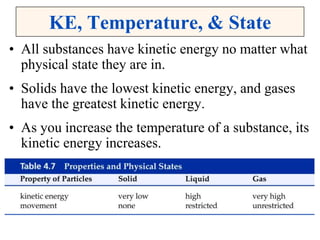
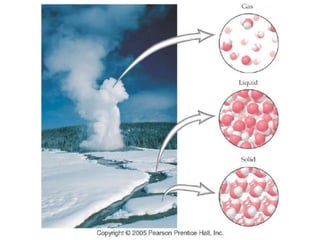
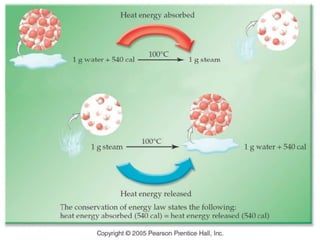
This document discusses matter, energy, and their various forms. It defines matter as anything that has mass and takes up space, and energy as the capacity to do work. There are different types of energy including mechanical, thermal, chemical, electrical, and nuclear. Energy can be transferred or converted between forms. The document also discusses the classification of matter into elements, compounds, and mixtures. Elements are made of only one type of atom, while compounds contain two or more elements chemically bonded together. Mixtures can be either homogeneous, containing a uniform composition, or heterogeneous.