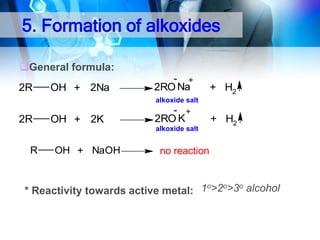
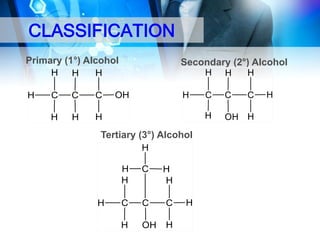
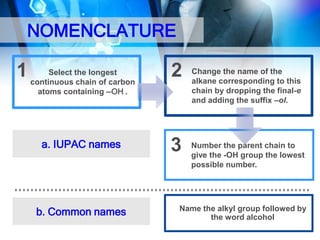
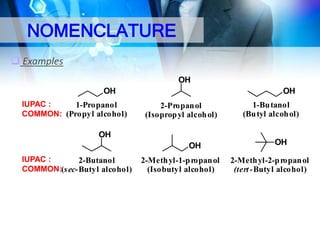
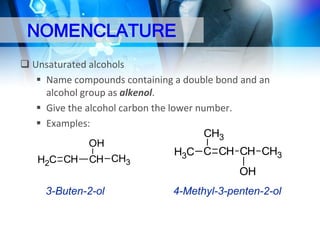
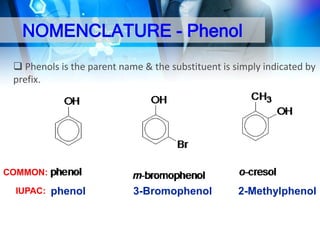
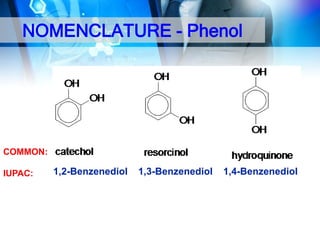
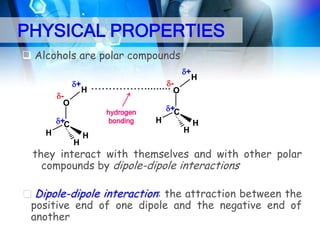
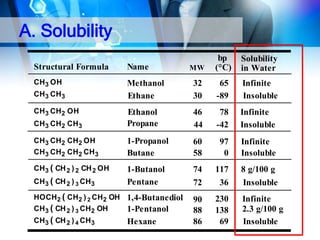
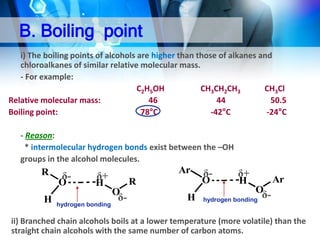
This document provides an introduction to alcohols. It begins by defining alcohols and phenols. It then discusses the classification of alcohols as primary, secondary, or tertiary based on the carbon atom bonded to the hydroxyl group. The document outlines some common nomenclature and naming conventions for alcohols and phenols. It also discusses some typical physical properties of alcohols like boiling point, solubility, and acidity due to hydrogen bonding. Finally, it briefly introduces some common preparation methods for alcohols like Grignard synthesis and hydrolysis of alkyl halides.














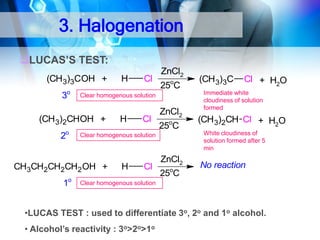
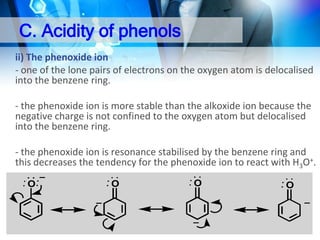
![C. Acidity
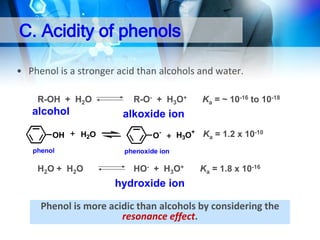
• Alcohol is weakly acidic.
• In aqueous solution, alcohol will donated its proton to water
molecule to give an alkoxide ion (R-O-).
R-OH + H2O
R-O- + H3O+
Ka = ~ 10-16 to 10-18
alkoxide ion
Example
CH3CH2-OH + H2O
CH3CH2-O- + H3O+
The acid-dissociation constant, Ka, of an alcohol is defined by the
equilibrium
K
a
Ka = [H3O+] [RO-]
R-OH + H2O
[ROH]
R-O- + H3O+
pKa = - log (Ka)
* More smaller the pKa
value, the alcohol is
more acidic](https://image.slidesharecdn.com/chapter1-alcohol-131023012037-phpapp01/85/Chapter-1-alcohol-25-320.jpg)
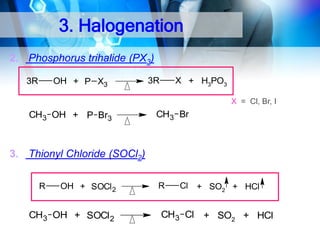
![C. Acidity
Alcohol is weakly acidic.
In aqueous solution, alcohol will donated its proton to water
molecule to give an alkoxide ion (R-O-).
CH3 O H + :O H
H
Ka =
+
CH3 O: + H O H
alkoxide ion
H
[ CH3 O-] [H3 O+ ]
[ CH3 OH]
–
= 1 0 - 15 .5
pKa = 1 5 .5
pKa decrease, acidity increase](https://image.slidesharecdn.com/chapter1-alcohol-131023012037-phpapp01/85/Chapter-1-alcohol-26-320.jpg)





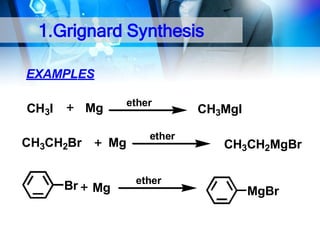
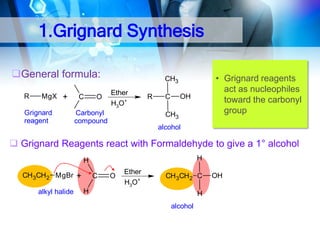
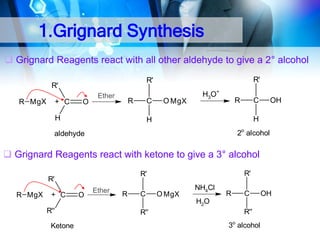
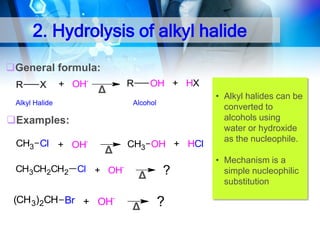

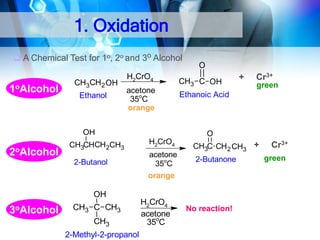
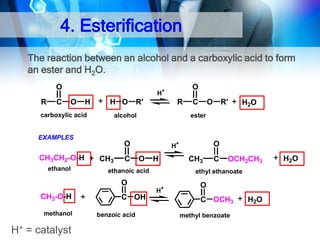
![1. Oxidation
Oxidation of 1°Alcohol to Aldehyde : RCH2-OH
[O]
RCHO
O
CH3CH 2OH + PCC
Ethanol
CH2CI2
o
25 C
CH3 C H
Ethanal
PCC: Pyridinium chlorochromate
Oxidation of 1°Alcohol to Carboxylic Acid : RCH2-OH
O
CH3CH 2 OH
Ethanol
H2CrO4
acetone
35oC
CH3 C OH
Ethanoic Acid
O
CH3CH 2 OH
Ethanol
KMnO4/ H+
CH3 C OH
Ethanoic Acid
[O]
RCOOH](https://image.slidesharecdn.com/chapter1-alcohol-131023012037-phpapp01/85/Chapter-1-alcohol-42-320.jpg)
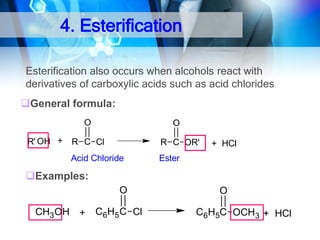
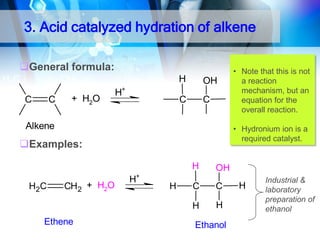
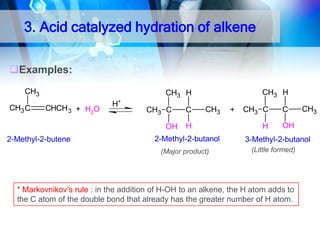
![1. Oxidation
O
OH
Oxidation of 2°Alcohol to Ketone :
R CHR'
[O]
O
OH
CH3CHCH2 CH3
H2CrO4
acetone
35oC
CH3CCH2CH3
2-Butanone
2-Butanol
O
OH
KMnO4/H+
cyclohexanol
cyclohexanone
R C R'](https://image.slidesharecdn.com/chapter1-alcohol-131023012037-phpapp01/85/Chapter-1-alcohol-43-320.jpg)