This document covers topics related to organic chemistry II, including:
- The general formula and nomenclature of carboxylic acids.
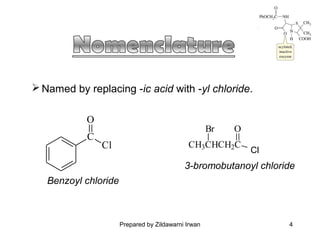
- The preparation of acid chlorides from carboxylic acids and their reactivity. Acid chlorides are more reactive than acids due to the chlorine withdrawing electron density.
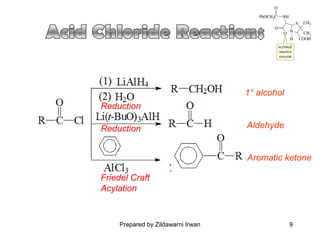
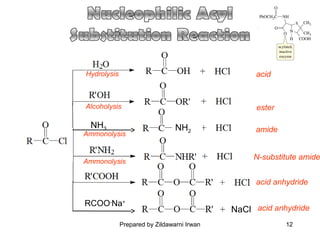
- Common reactions of acid chlorides including reduction, Friedel-Crafts acylation, and nucleophilic acyl substitution such as hydrolysis, ammonolysis, and alcoholysis.