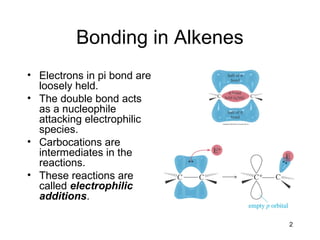
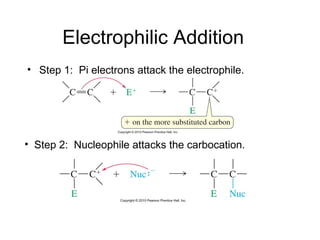
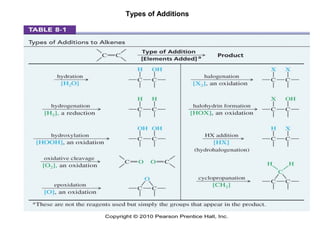
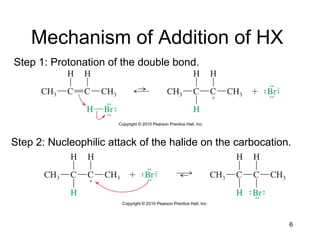
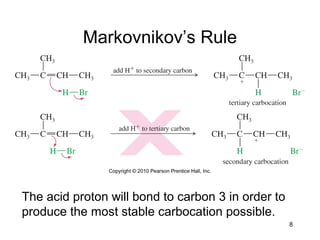
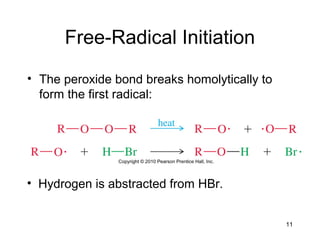
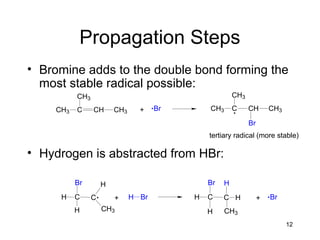
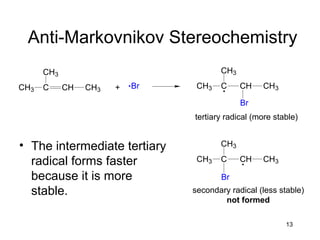
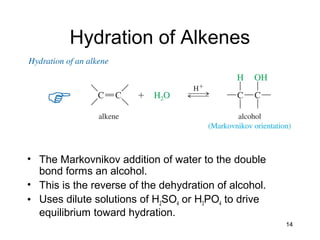
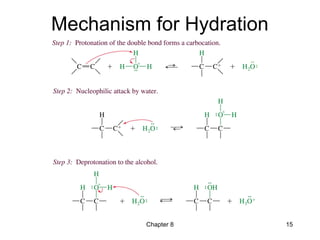
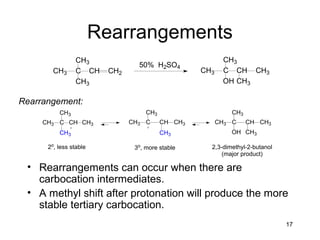
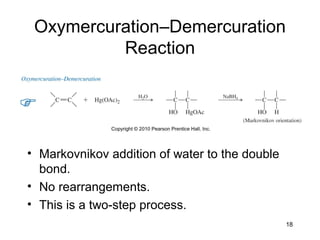
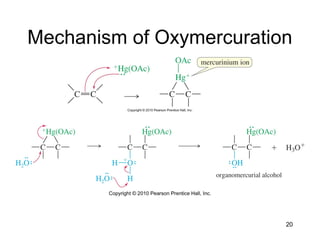
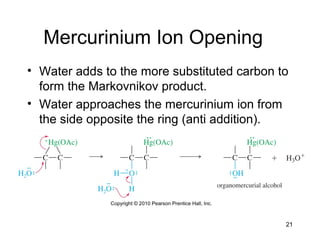
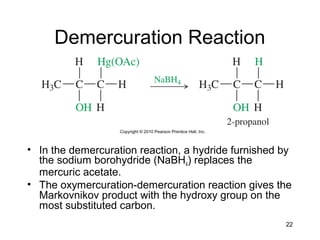
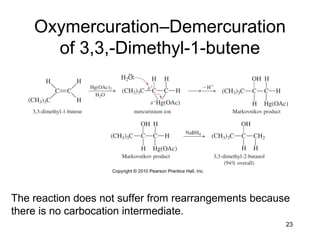
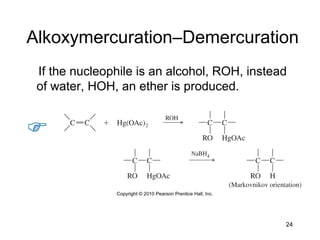
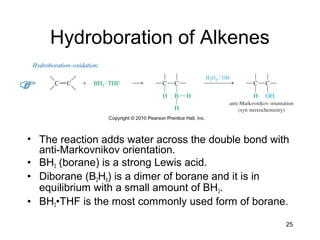
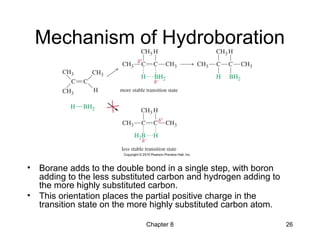
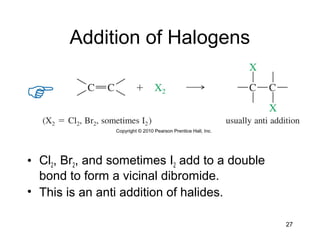
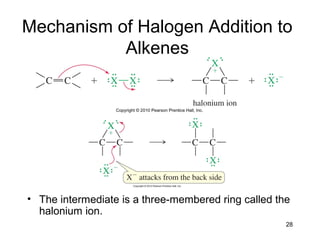
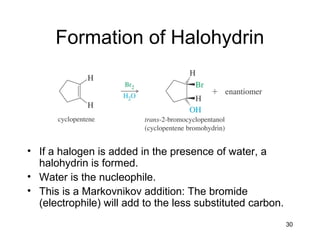
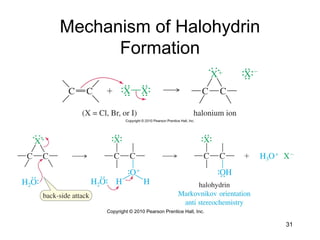
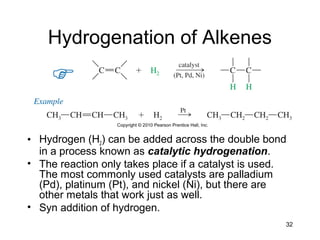
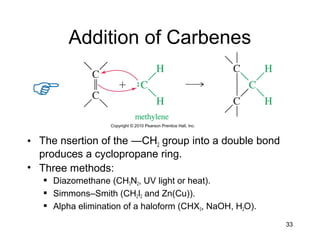
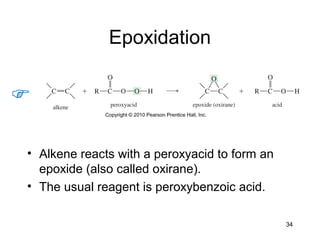
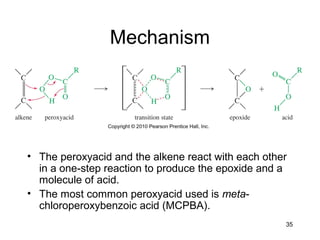
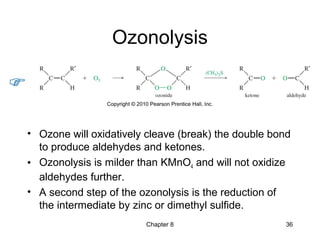
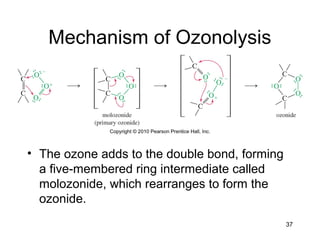
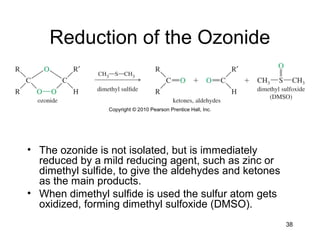
Electrophilic additions involve reactions of alkenes where the pi electrons in the double bond attack an electrophile. There are several types of additions including addition of HX, halogens, water, alcohols, and hydroboration. The mechanism typically involves formation of a carbocation intermediate that is then attacked by the nucleophile. Addition occurs regioselectively according to Markovnikov's rule, favoring the most stable carbocation. Exceptions include free radical additions, which give the anti-Markovnikov product. Oxymercuration-demercuration and hydroboration allow for Markovnikov addition without rearrangements.