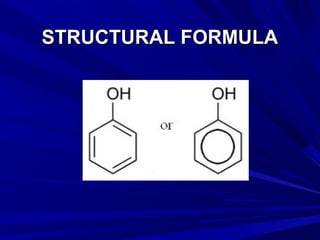
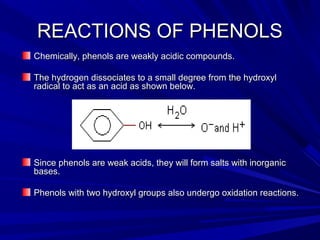
Phenols are chemical compounds that contain a hydroxyl group attached to an aromatic hydrocarbon group. Phenols are hydroxyl derivatives of hydrocarbons where a hydrogen on the benzene ring is replaced by a hydroxyl group. Phenols react in various ways including forming salts with bases, undergoing oxidation, and reacting with bromine water, nitric acid, iron chloride, and other compounds. Phenols have medical uses as keratolytics, antipruritics, and disinfectants due to their caustic effects on tissues.