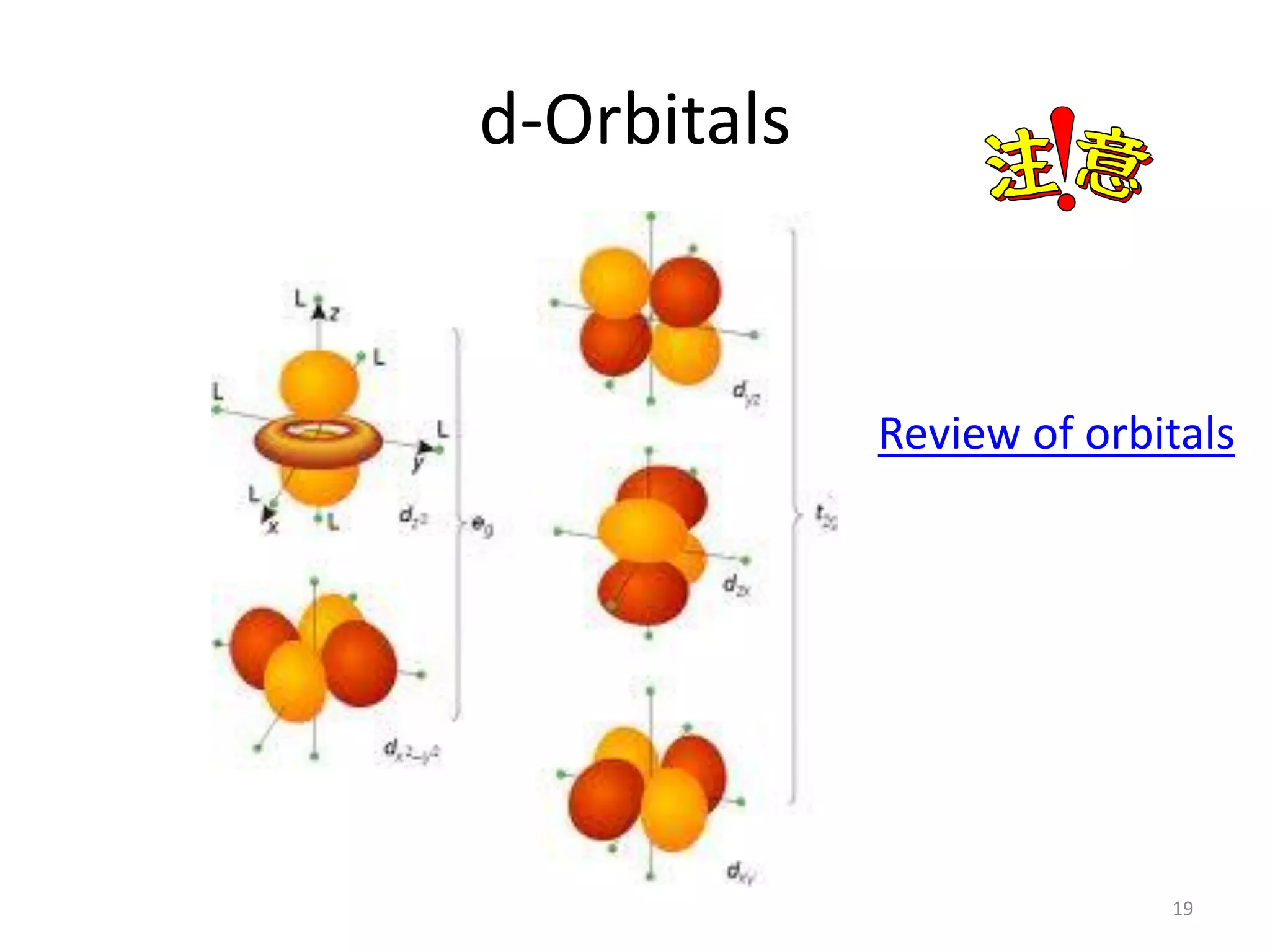
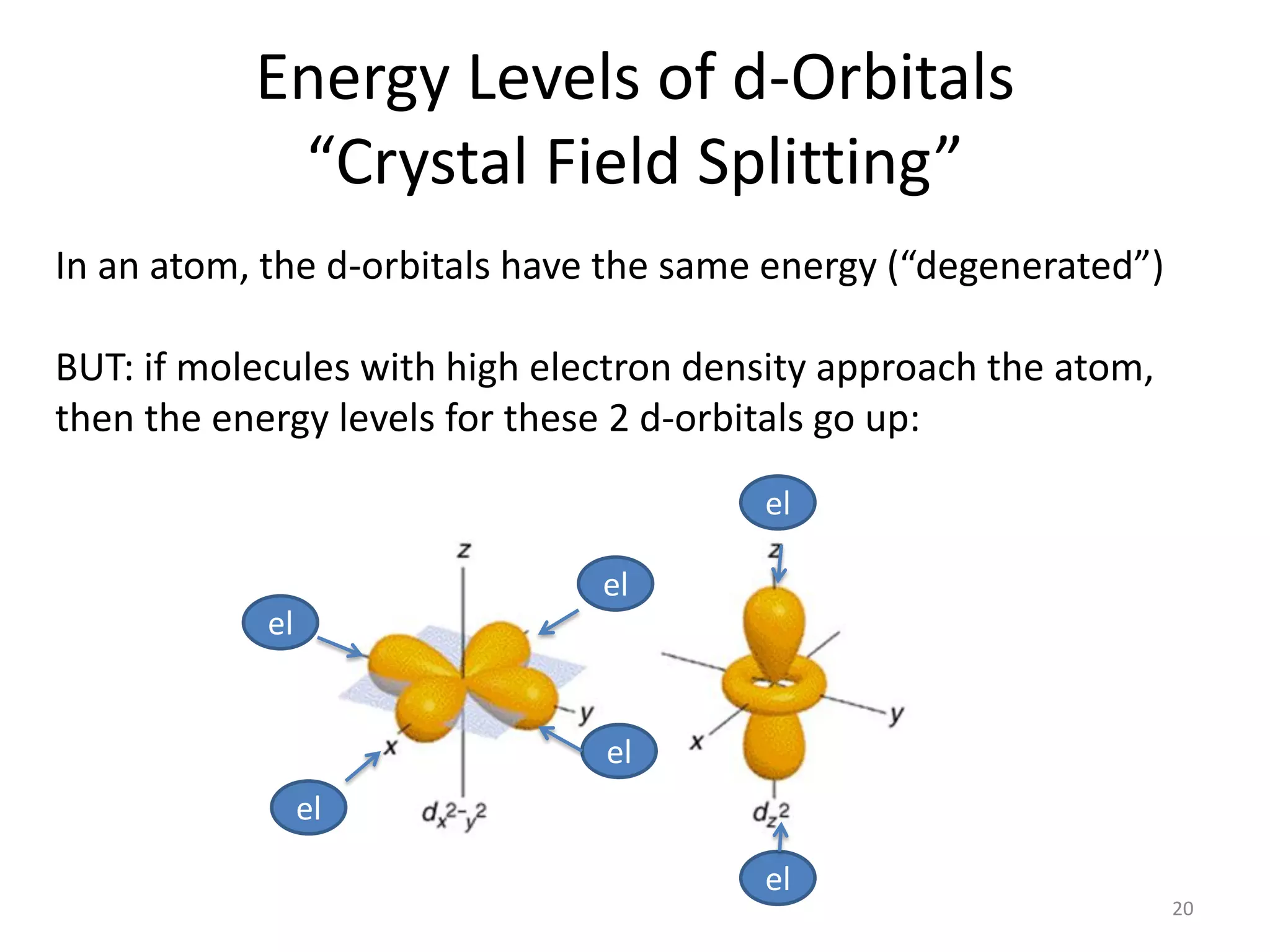
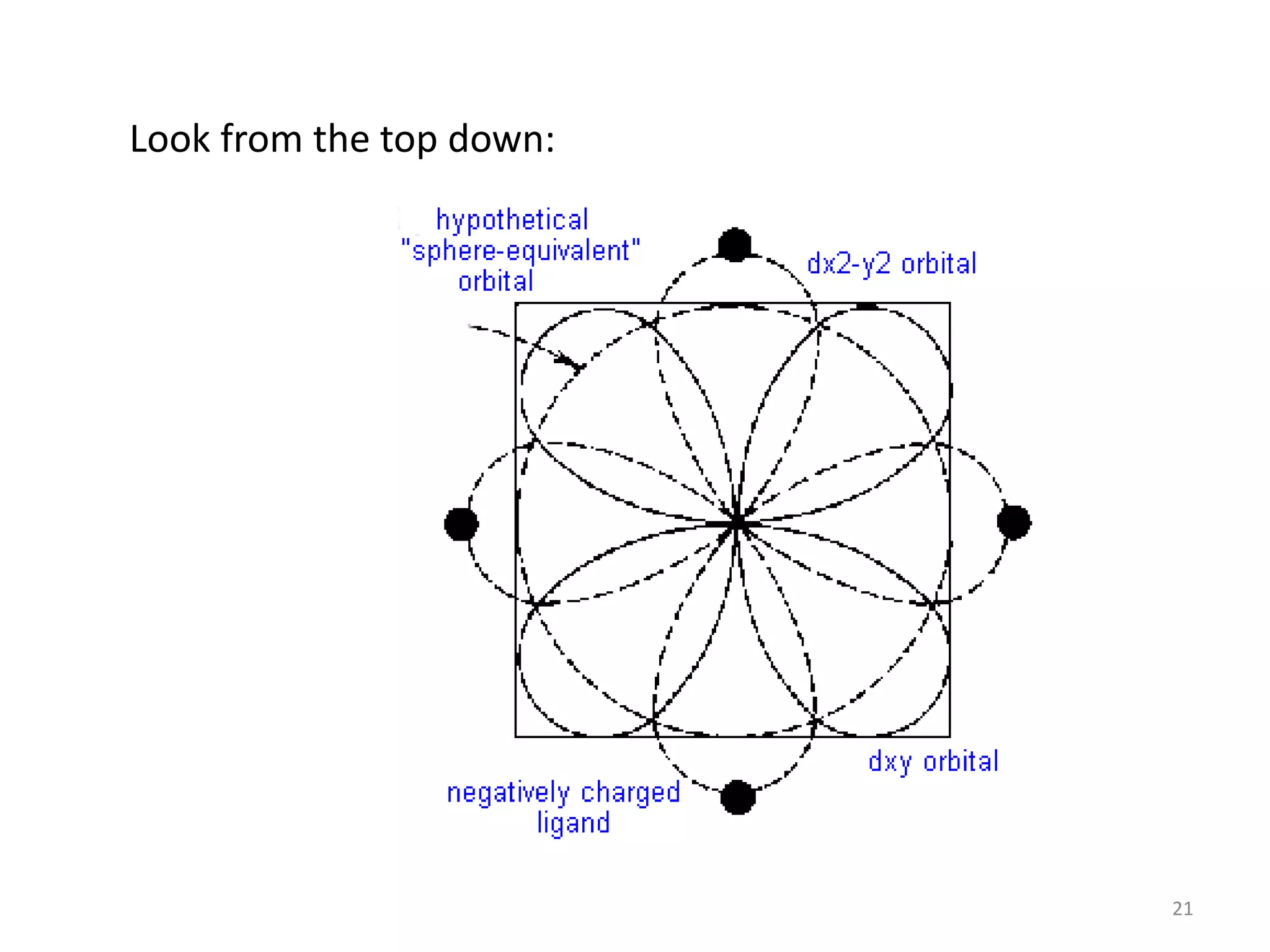
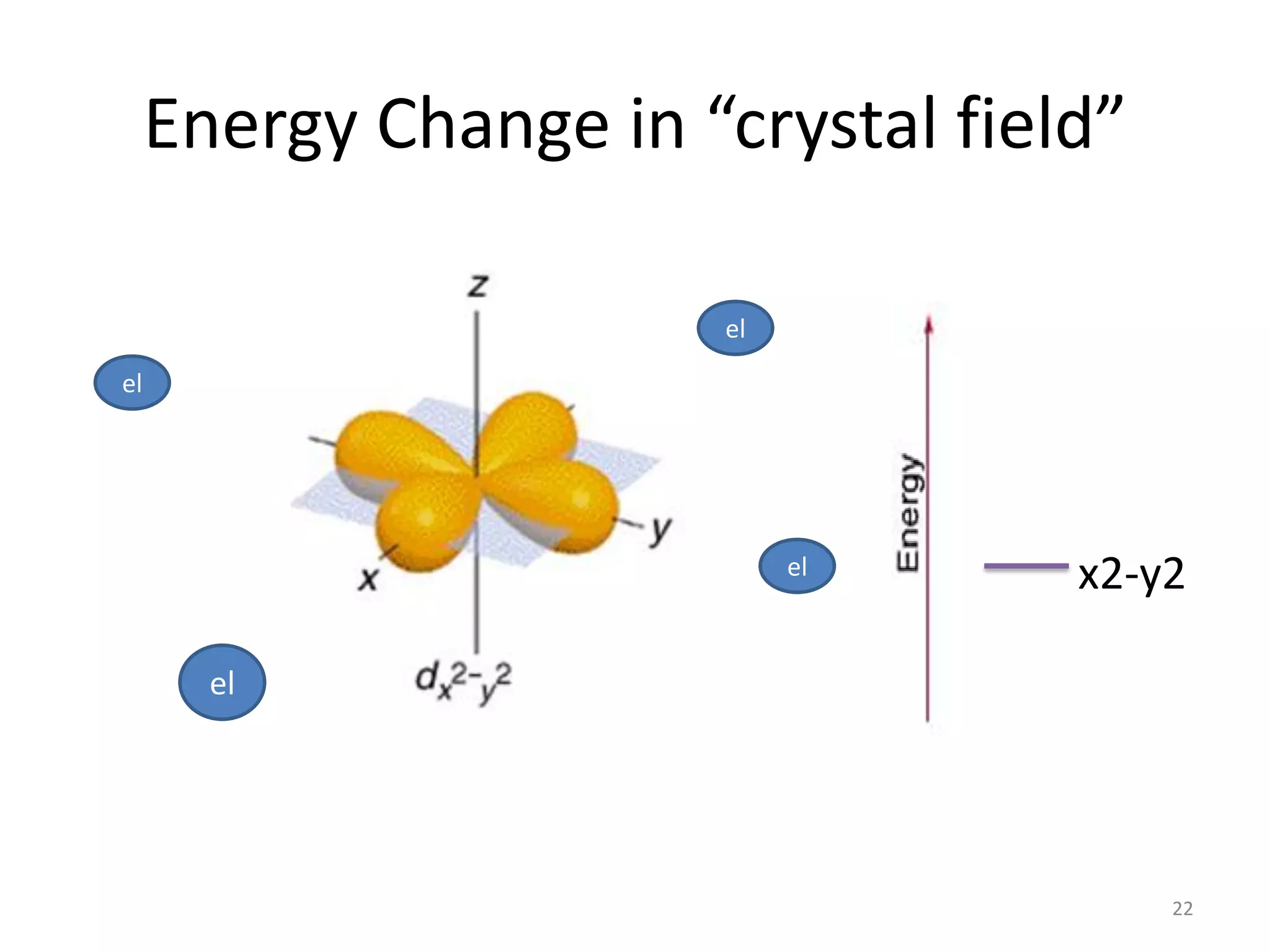
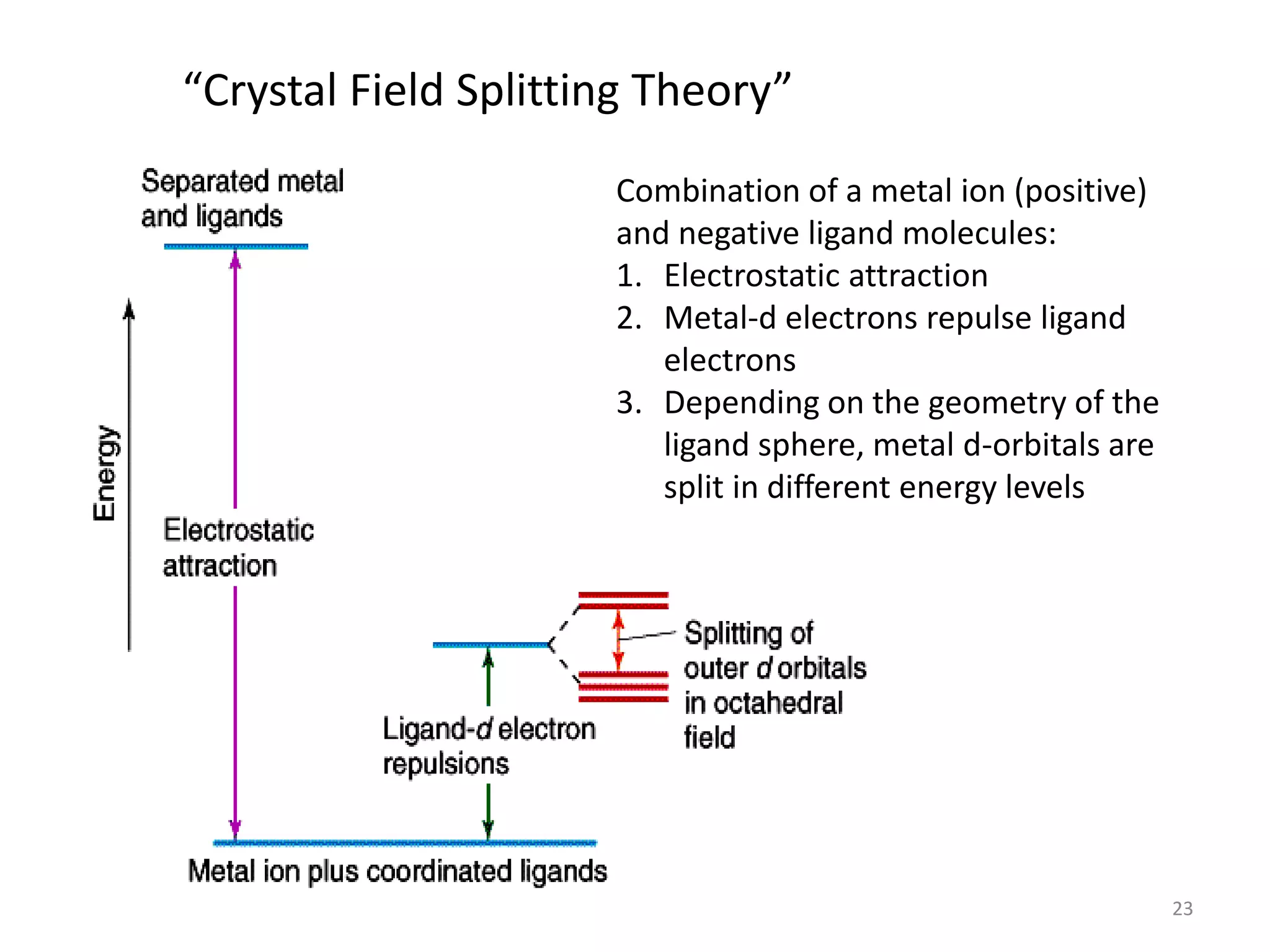
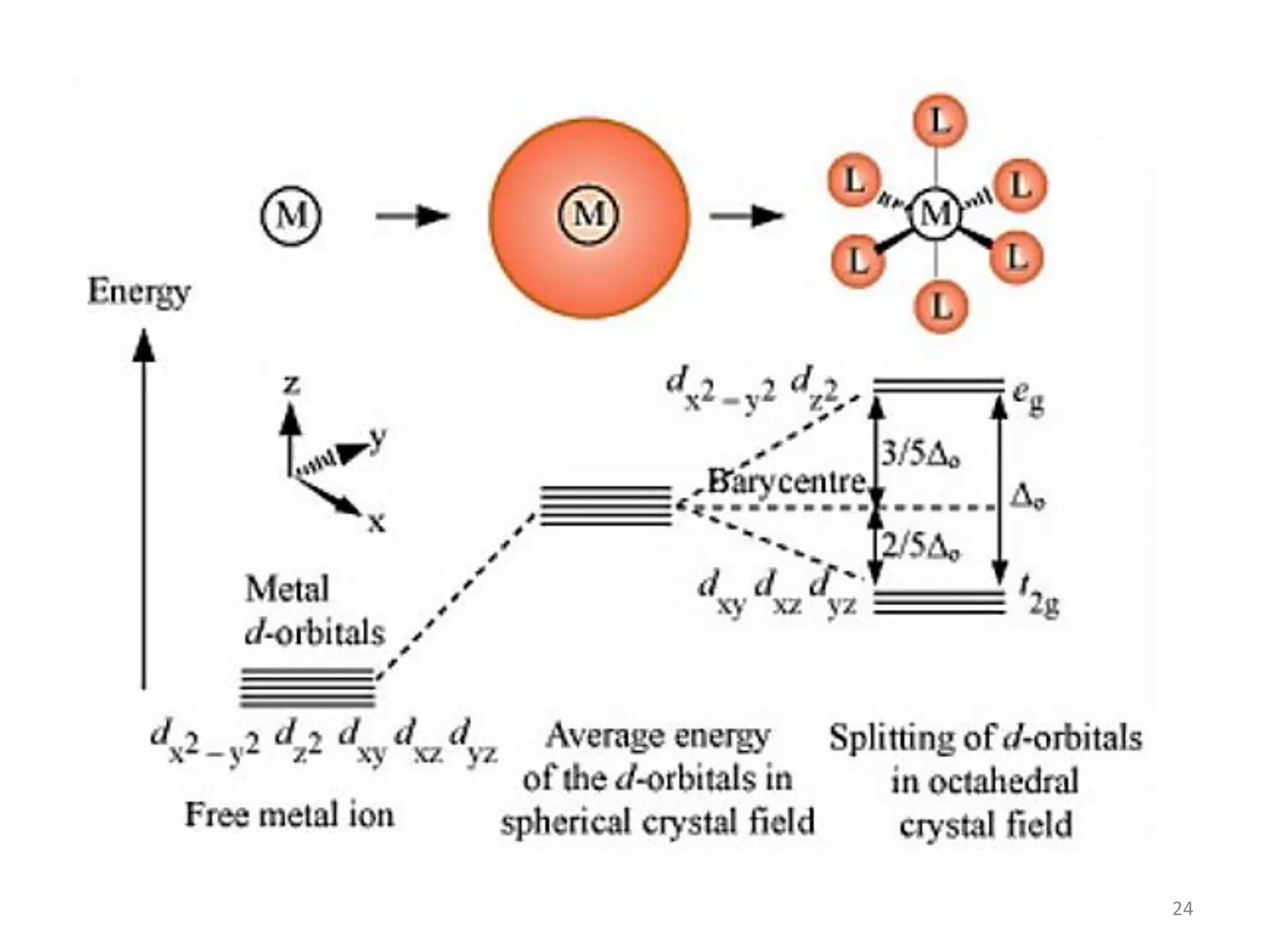
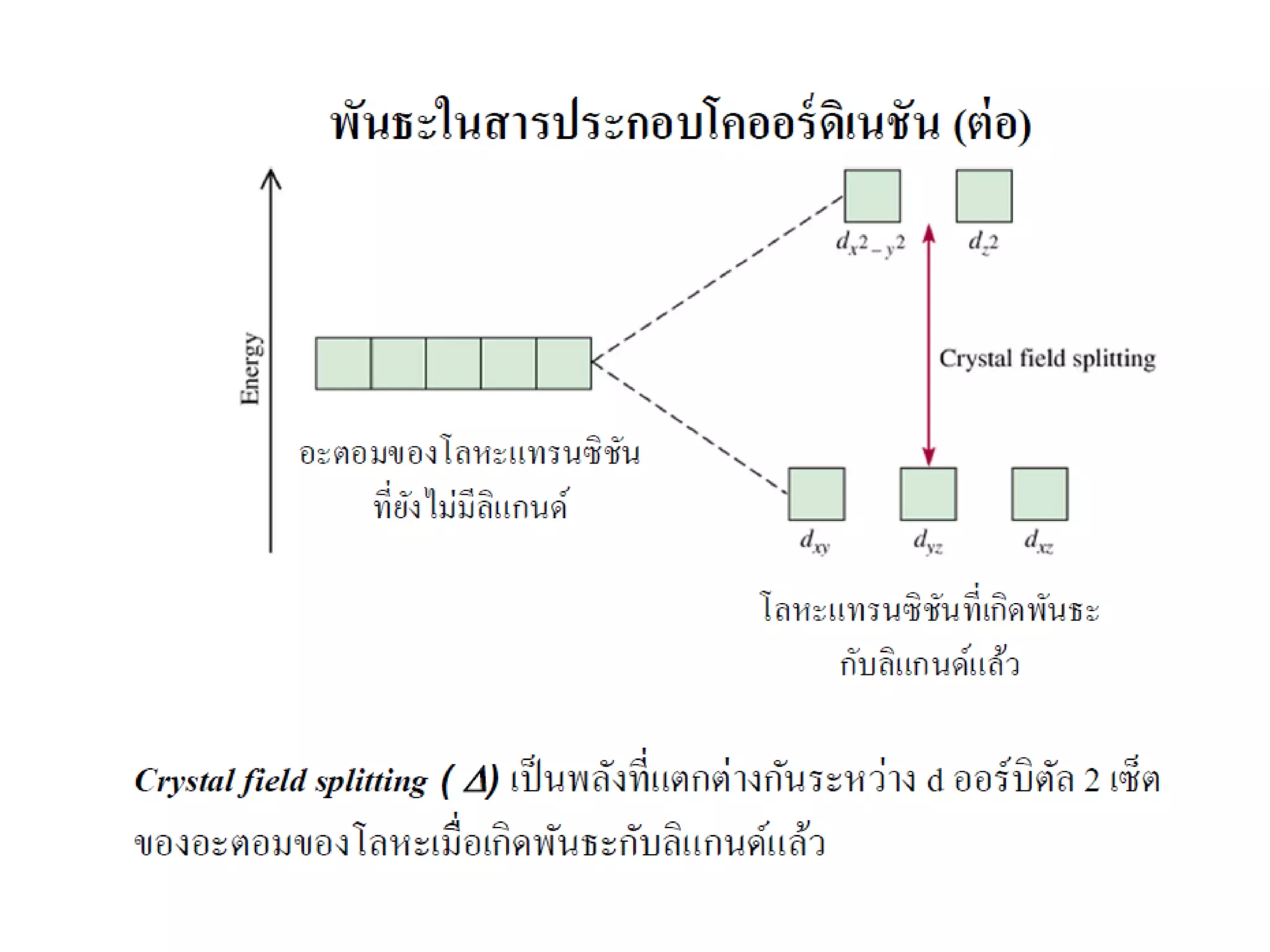
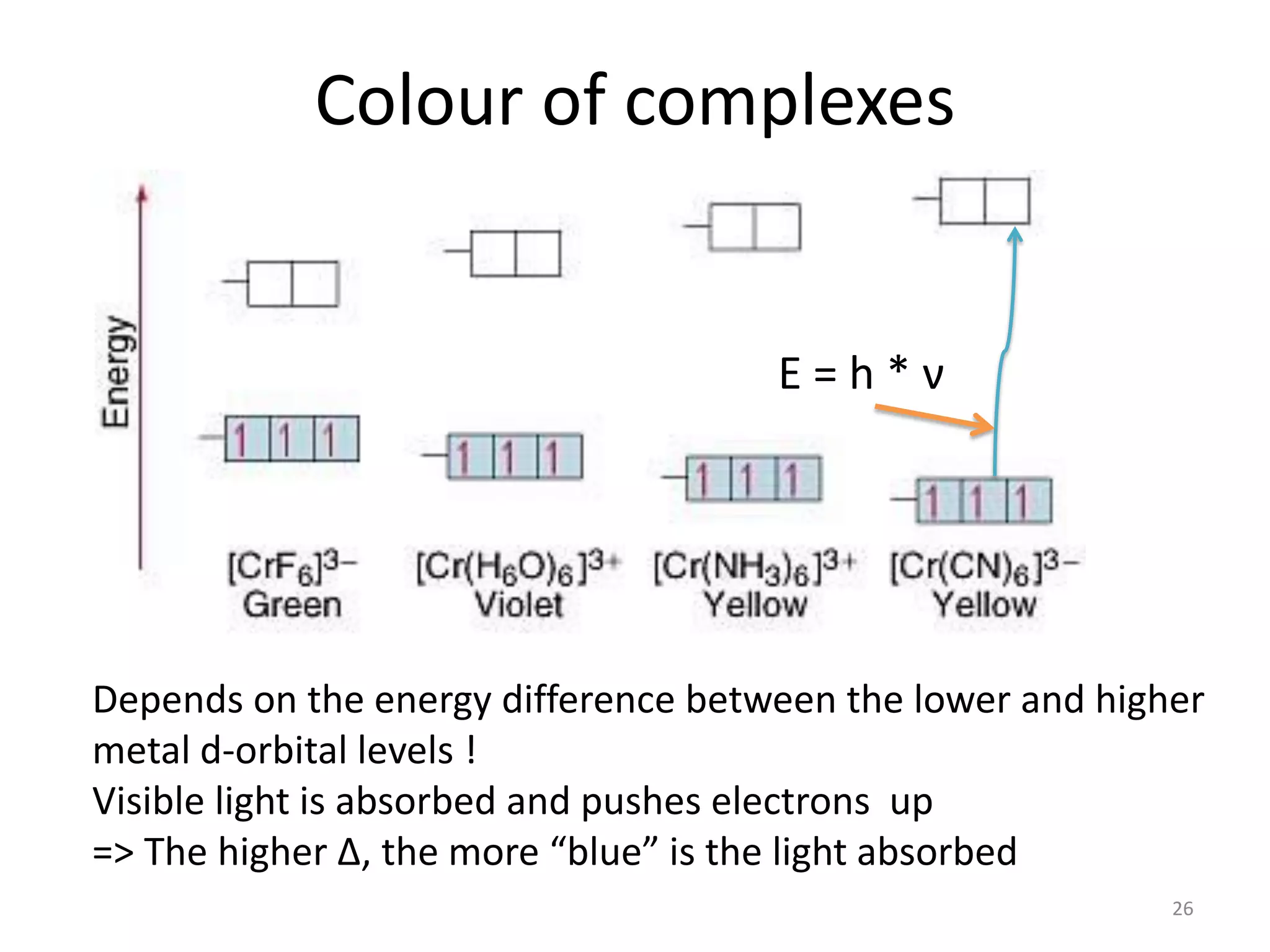
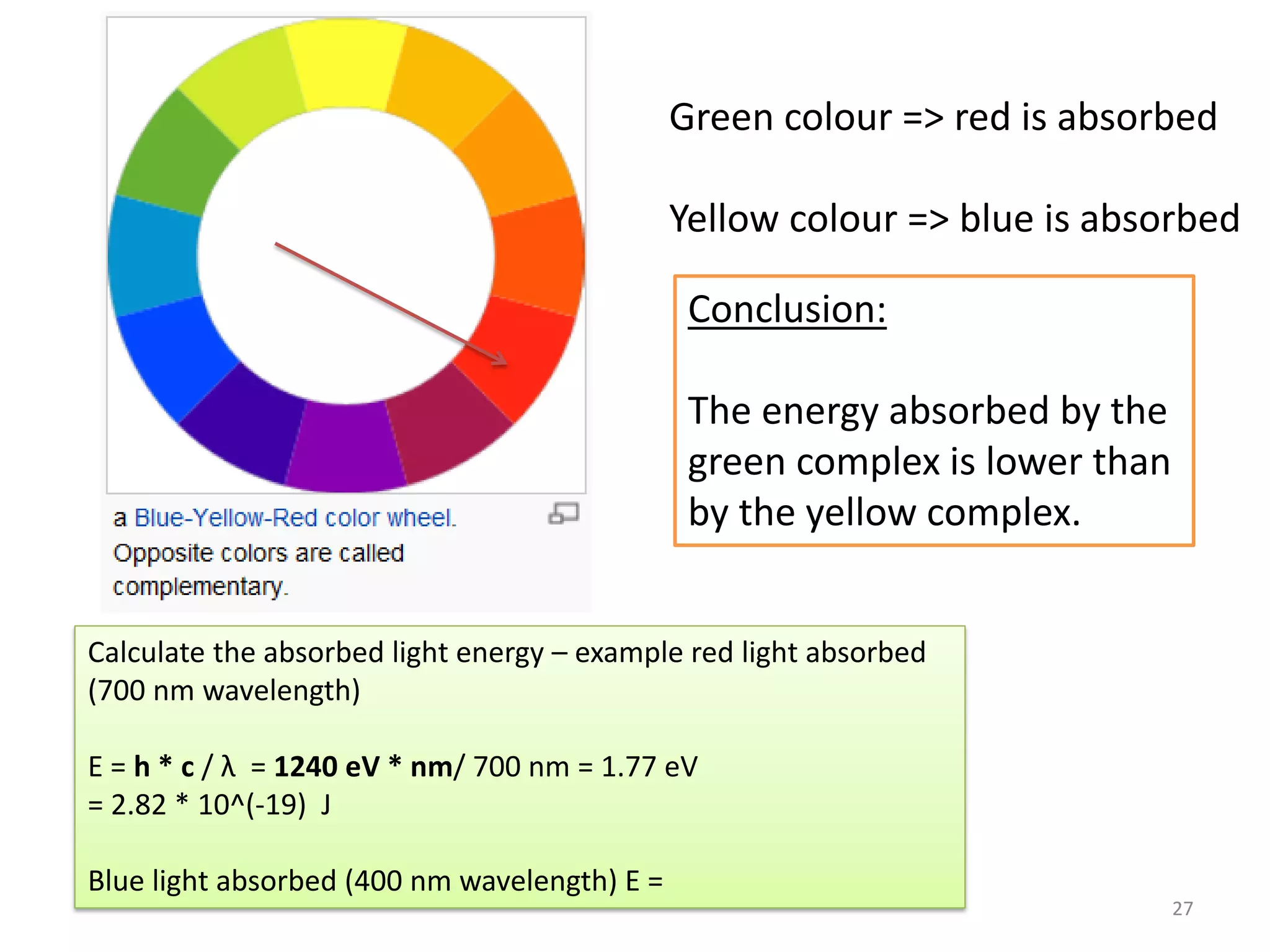
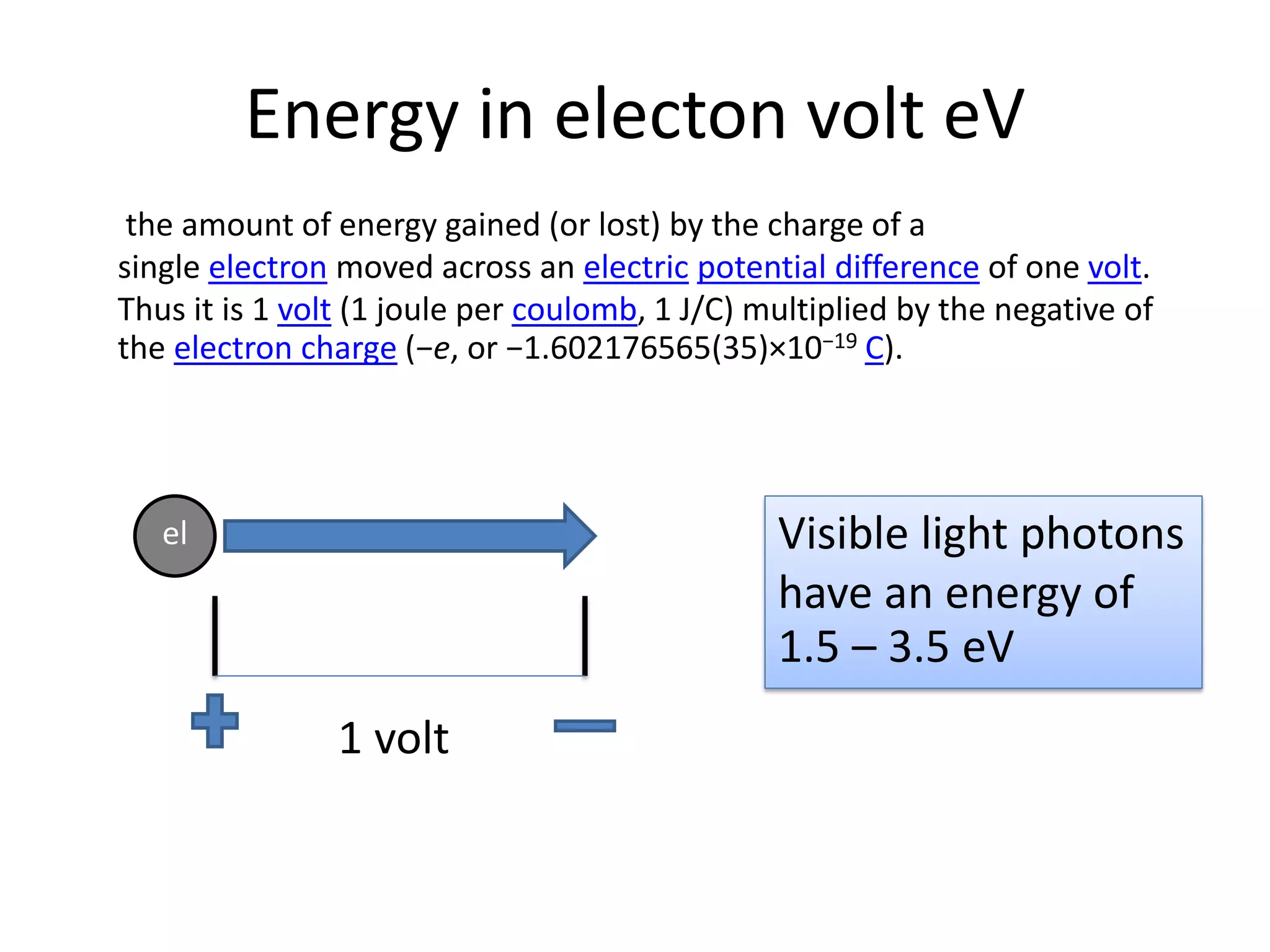
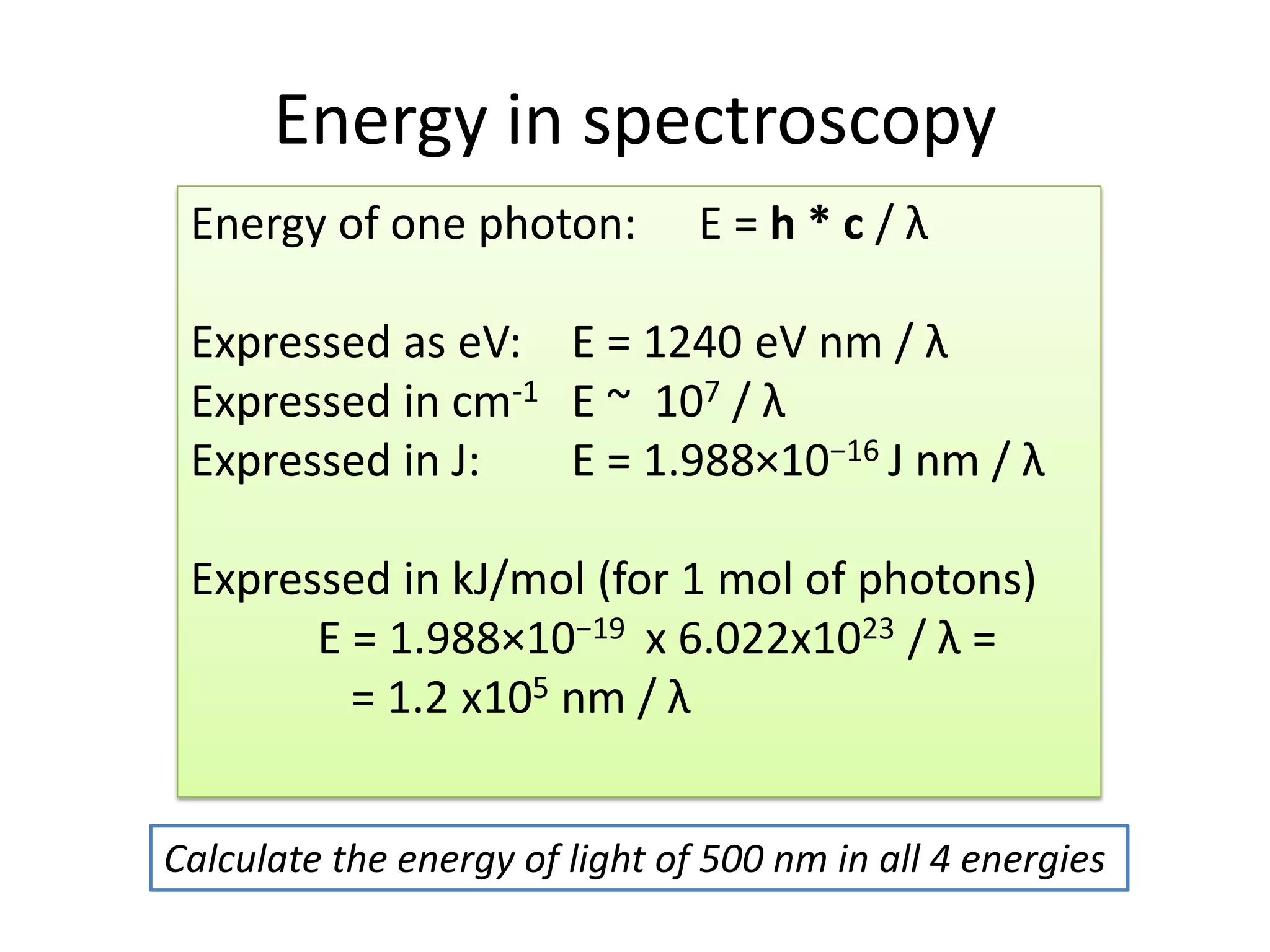
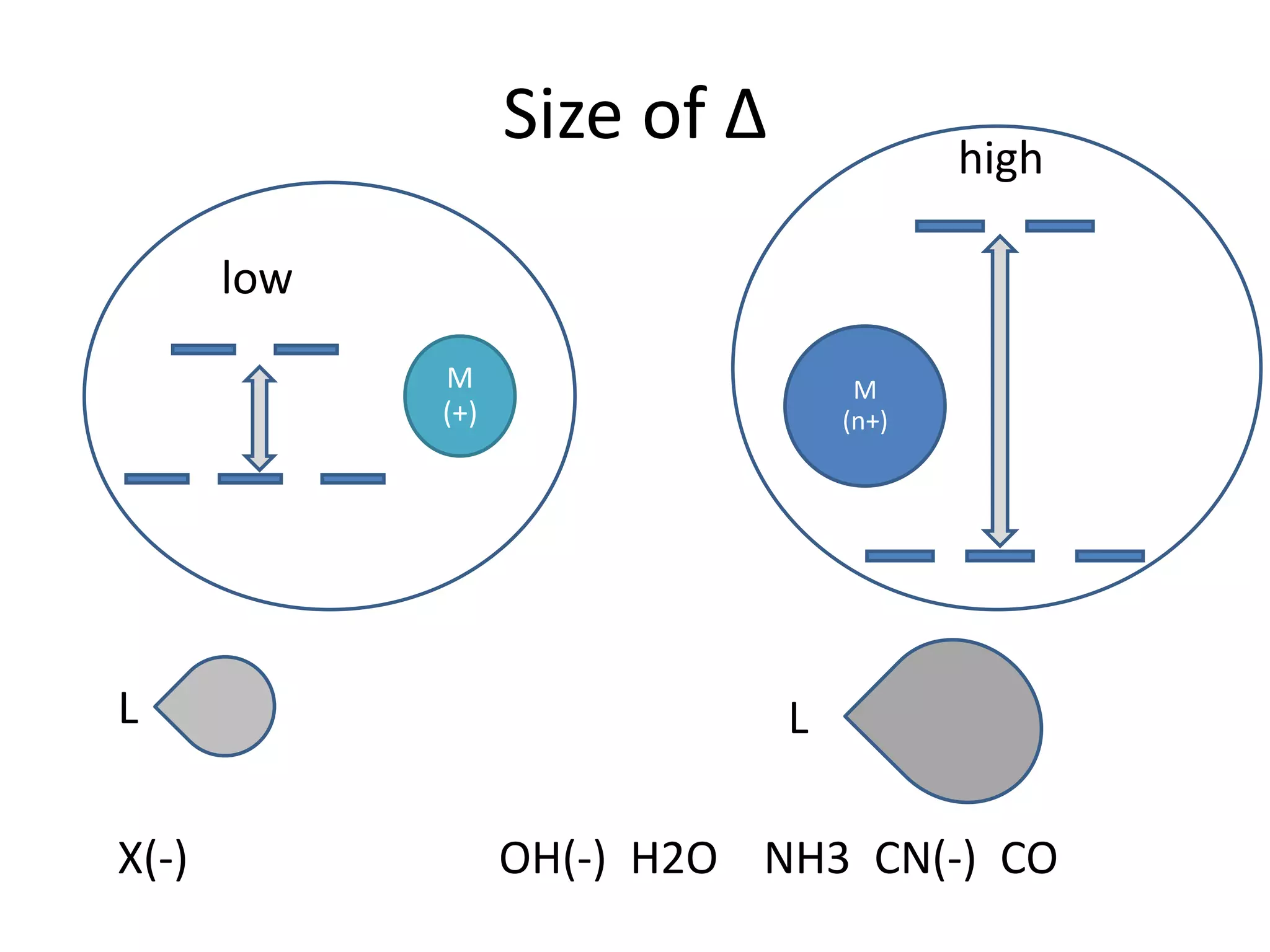
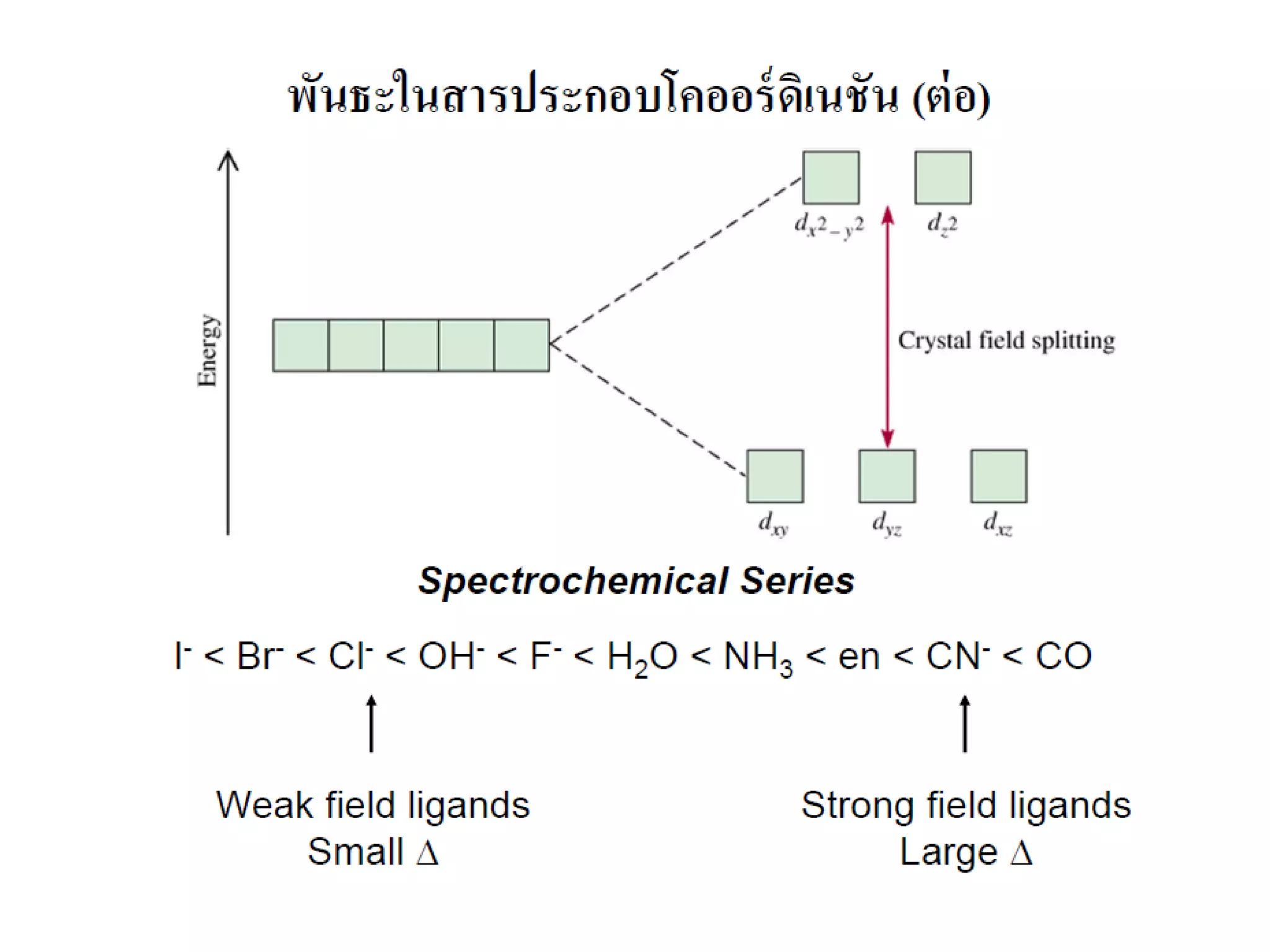
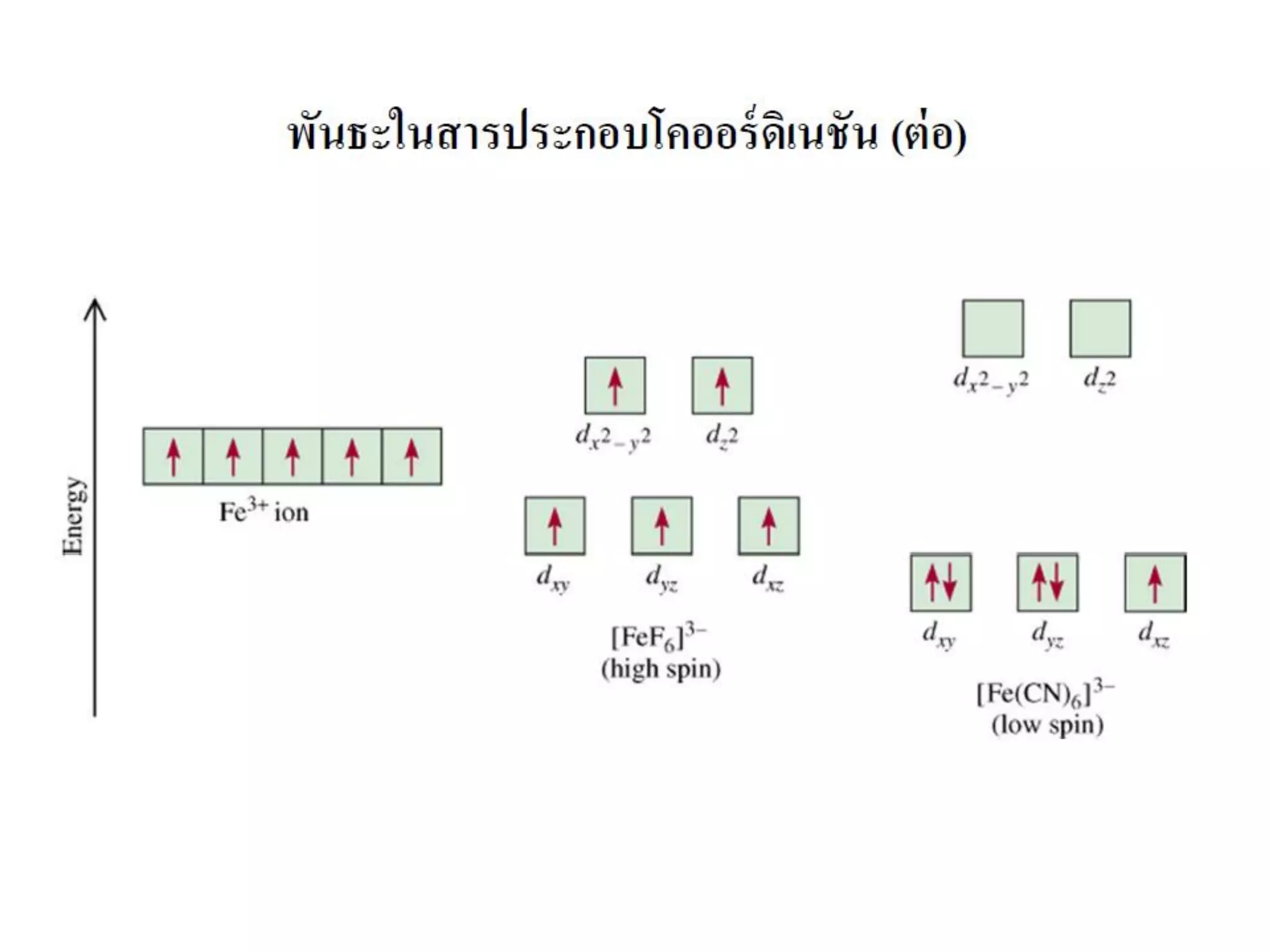
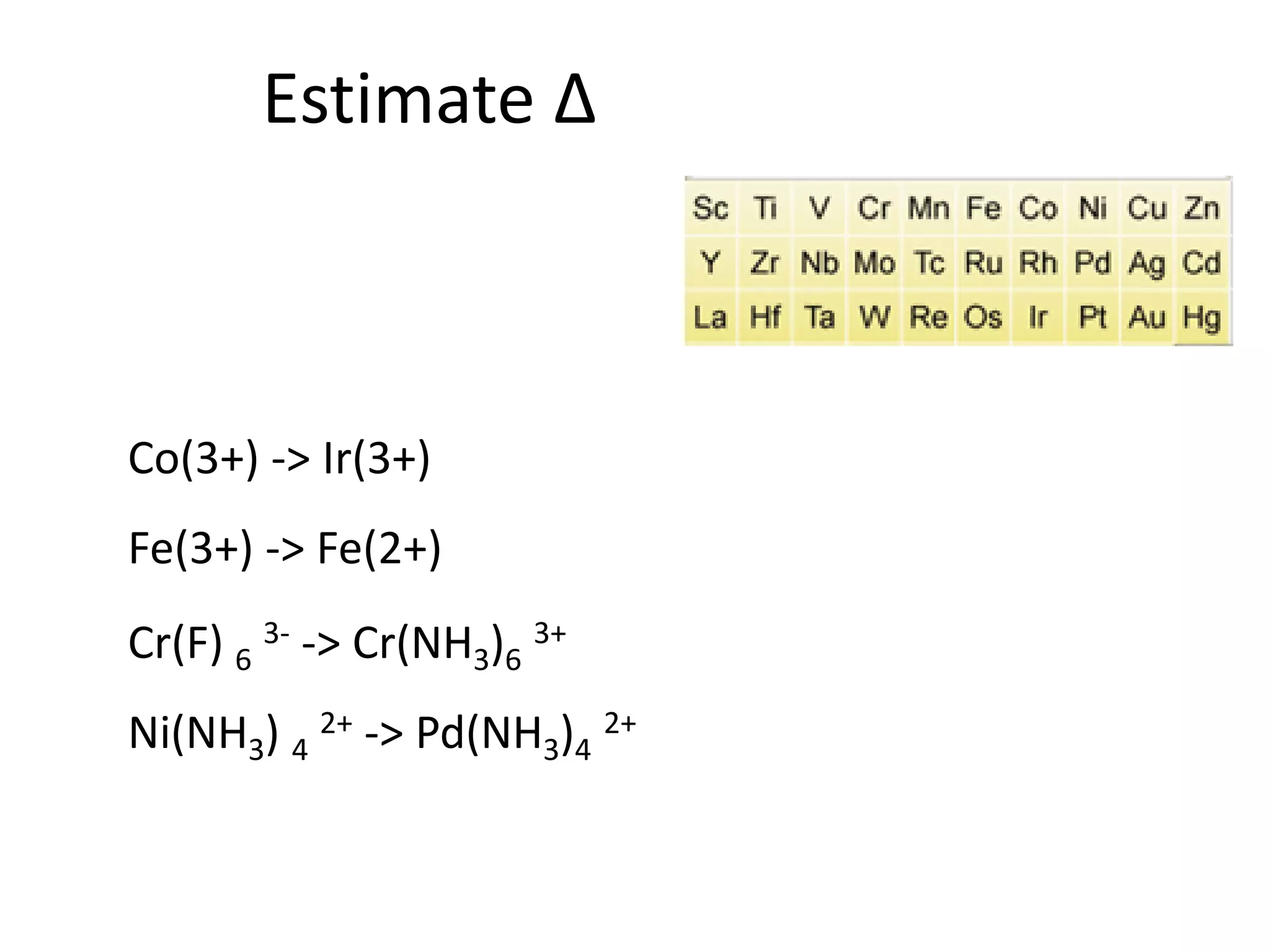
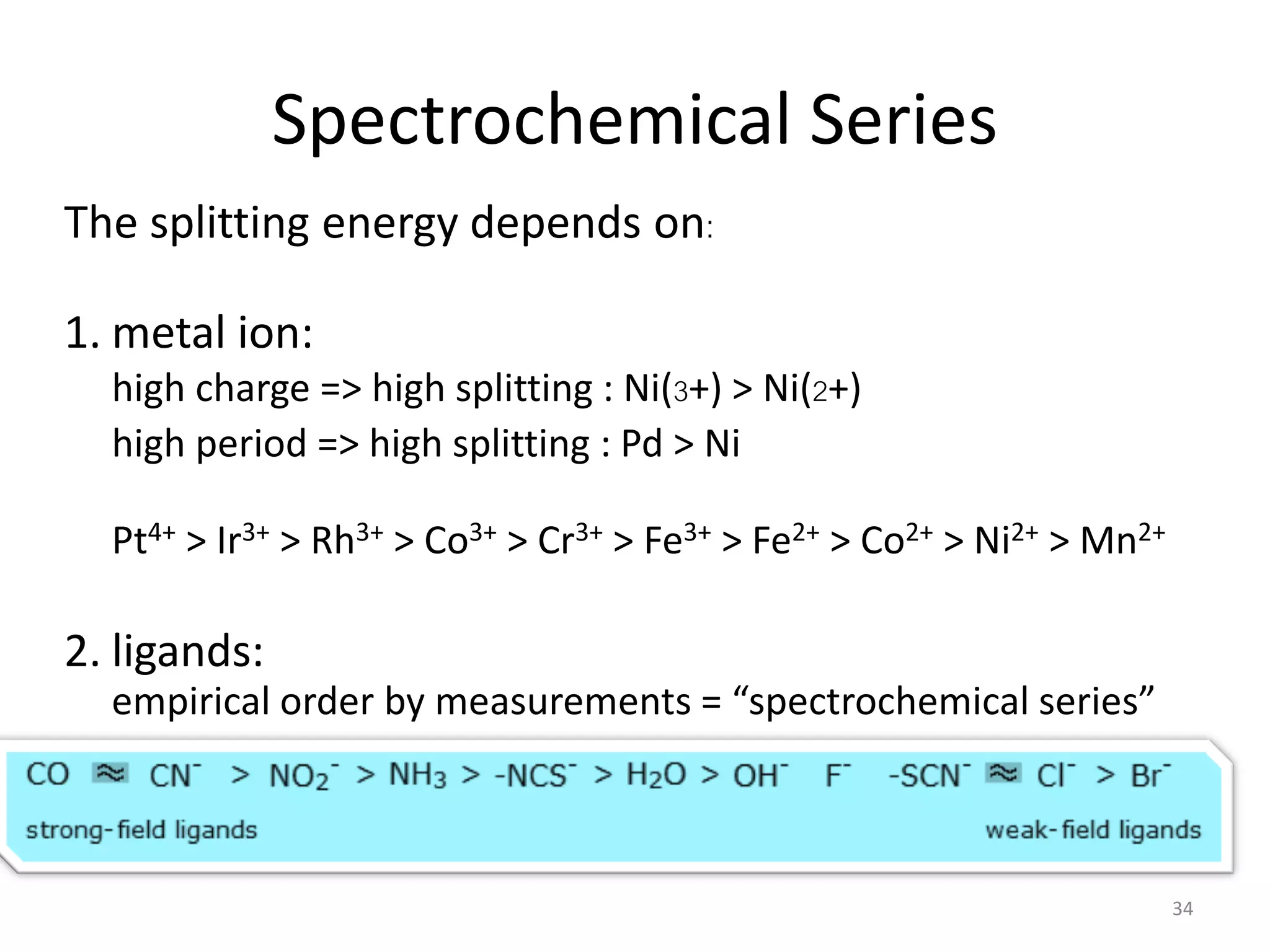
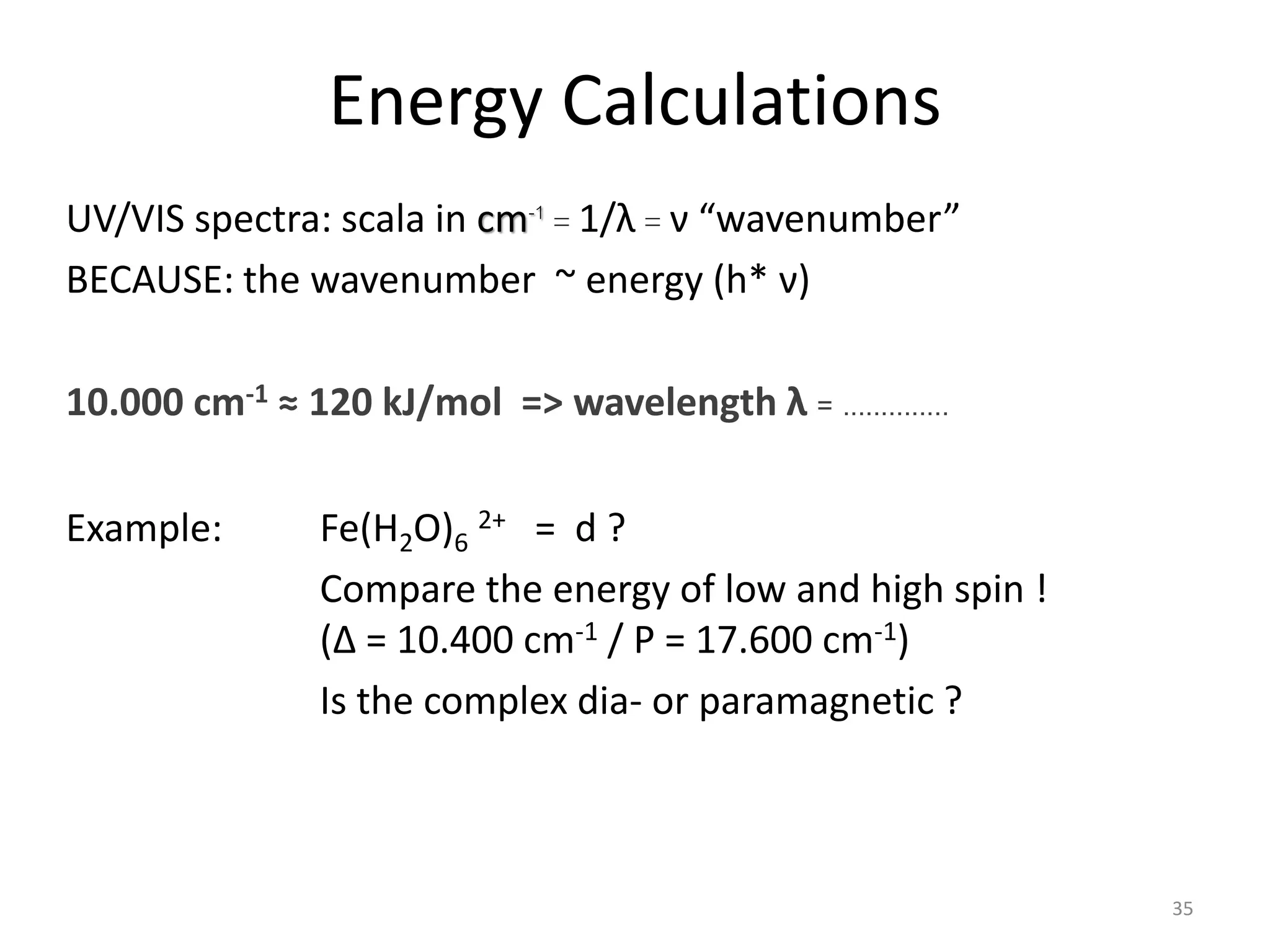
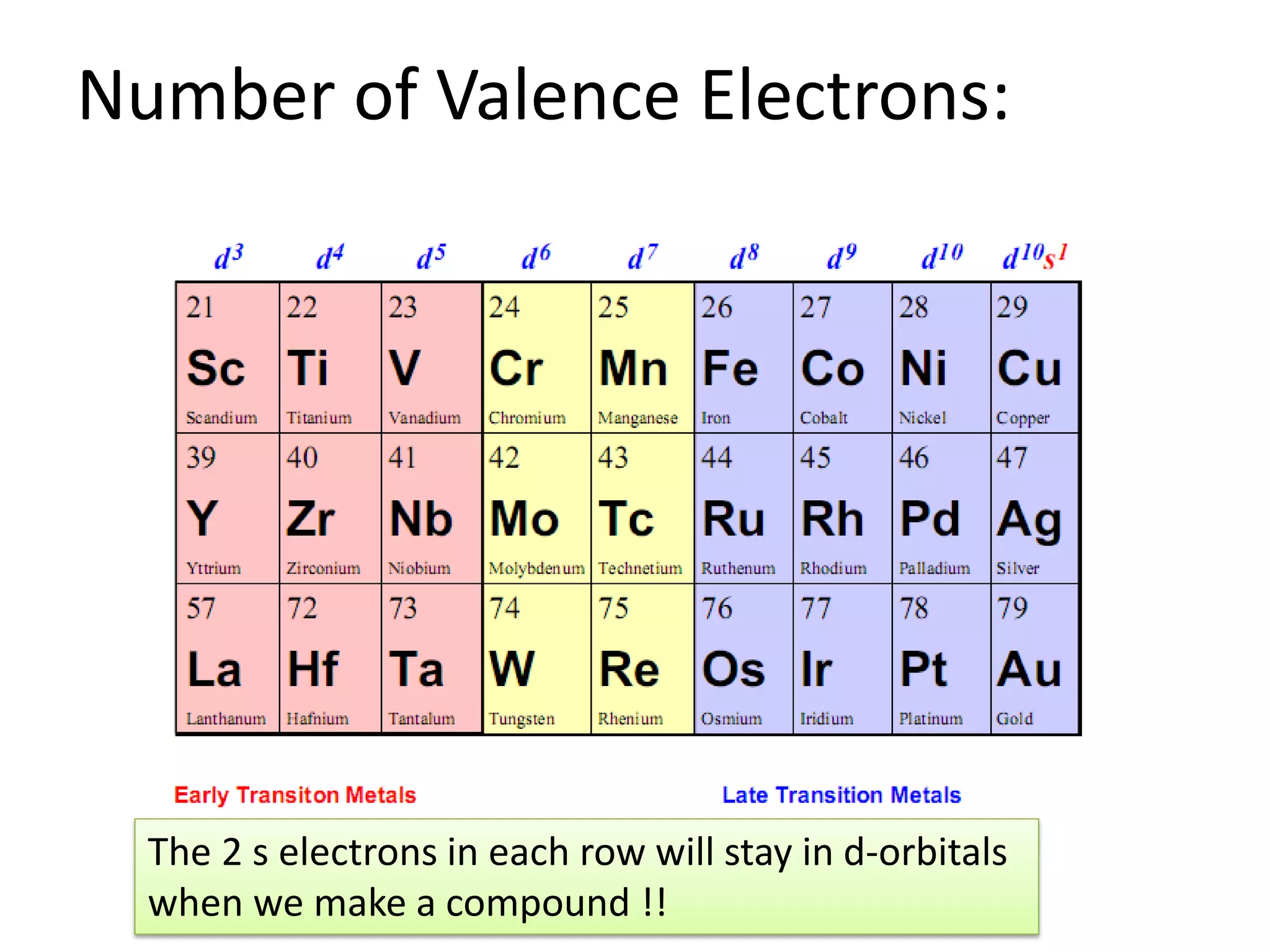
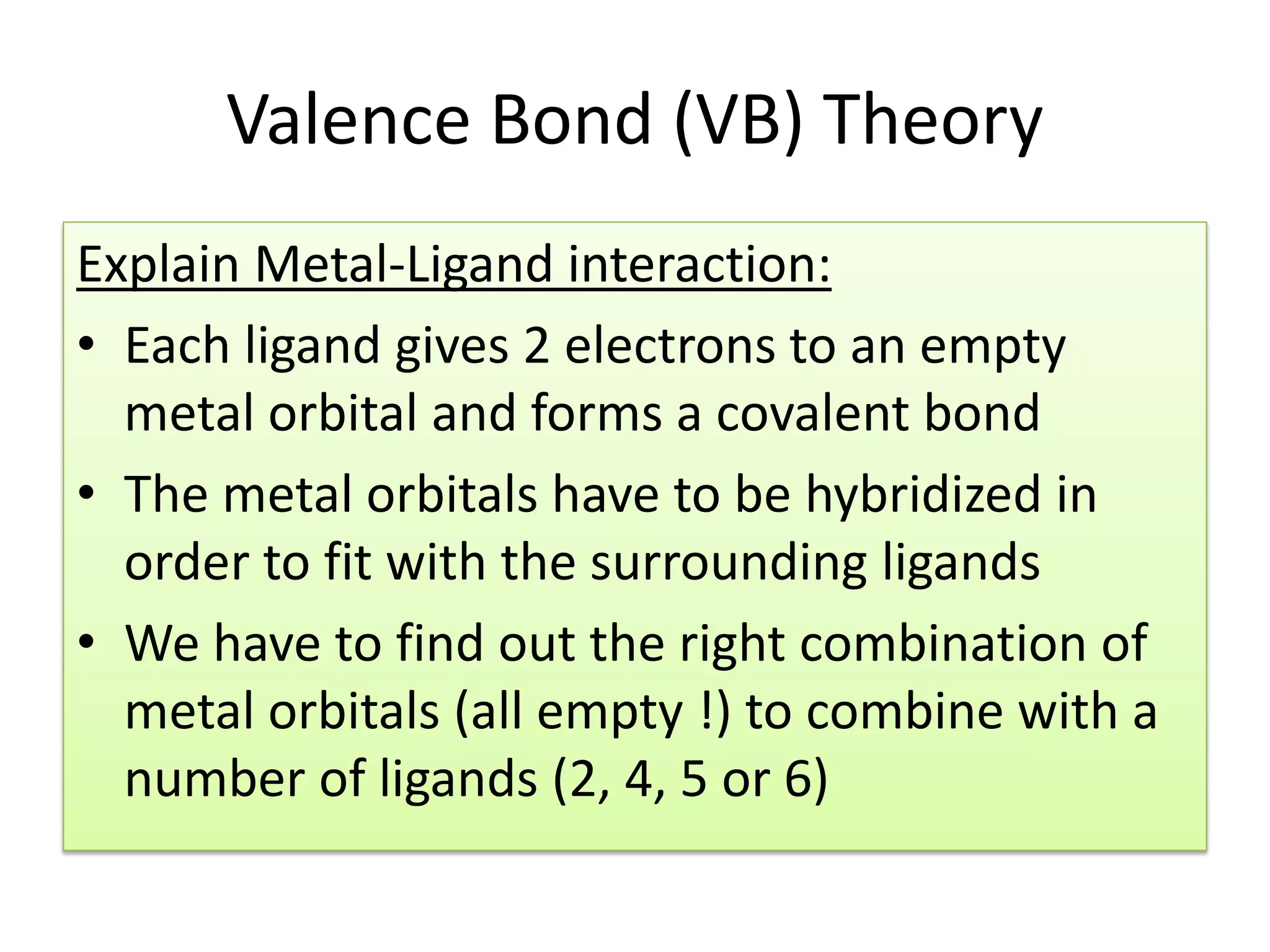
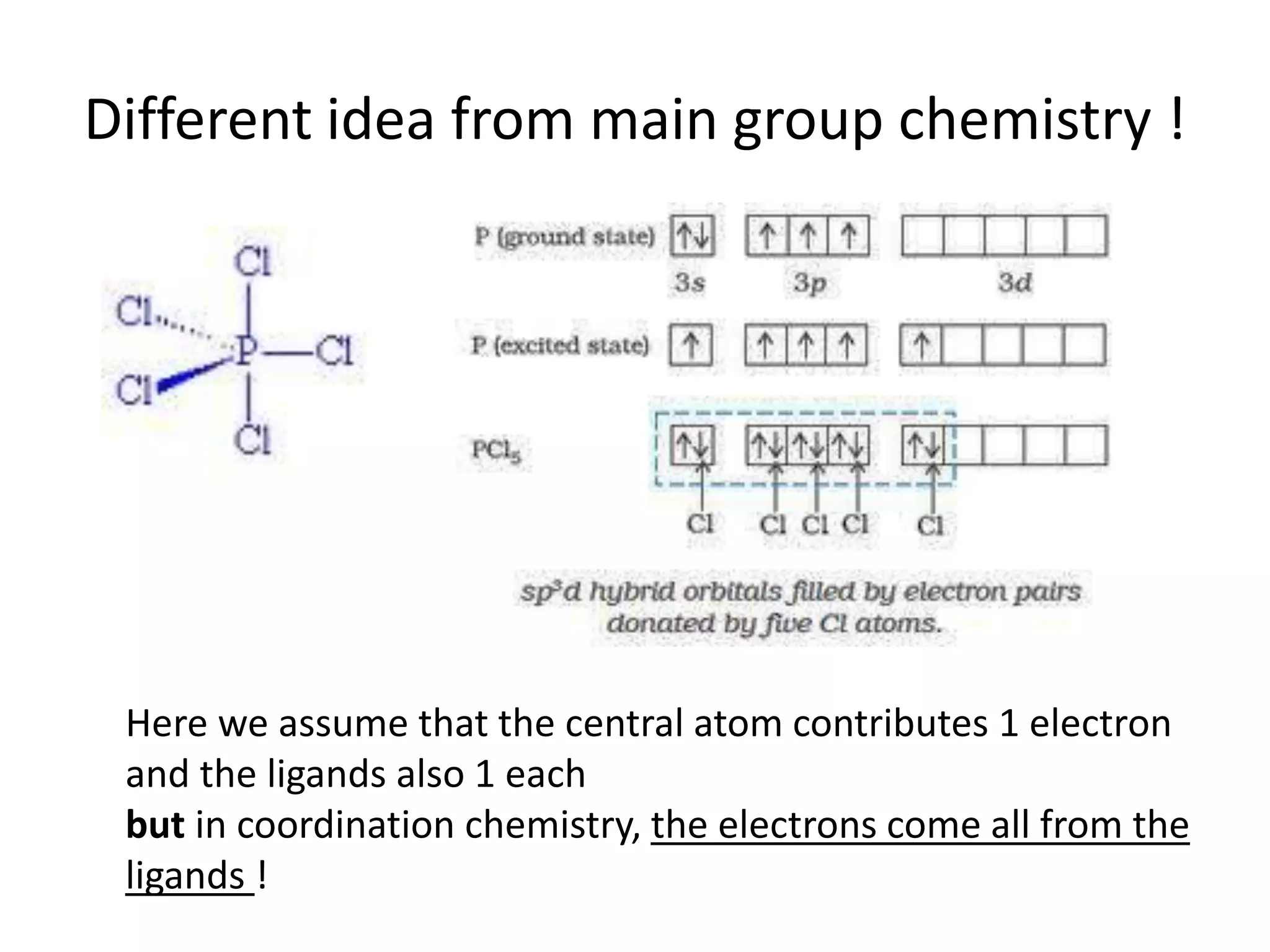
This document provides an overview of bonding models for transition metal compounds. It discusses valence bond theory and how ligands donate electrons to empty metal orbitals to form bonds. Crystal field theory and ligand field theory are introduced to explain how the electronic structure of transition metals is affected by ligands in their coordination sphere. The ligands create a crystal field that splits the degenerate d-orbitals of the metal into different energy levels. The size of this splitting depends on factors like the metal ion and type of ligands. Spectroscopic properties of complexes, such as color, are determined by the energy gap between these split d-orbital levels.



![Possible answers:
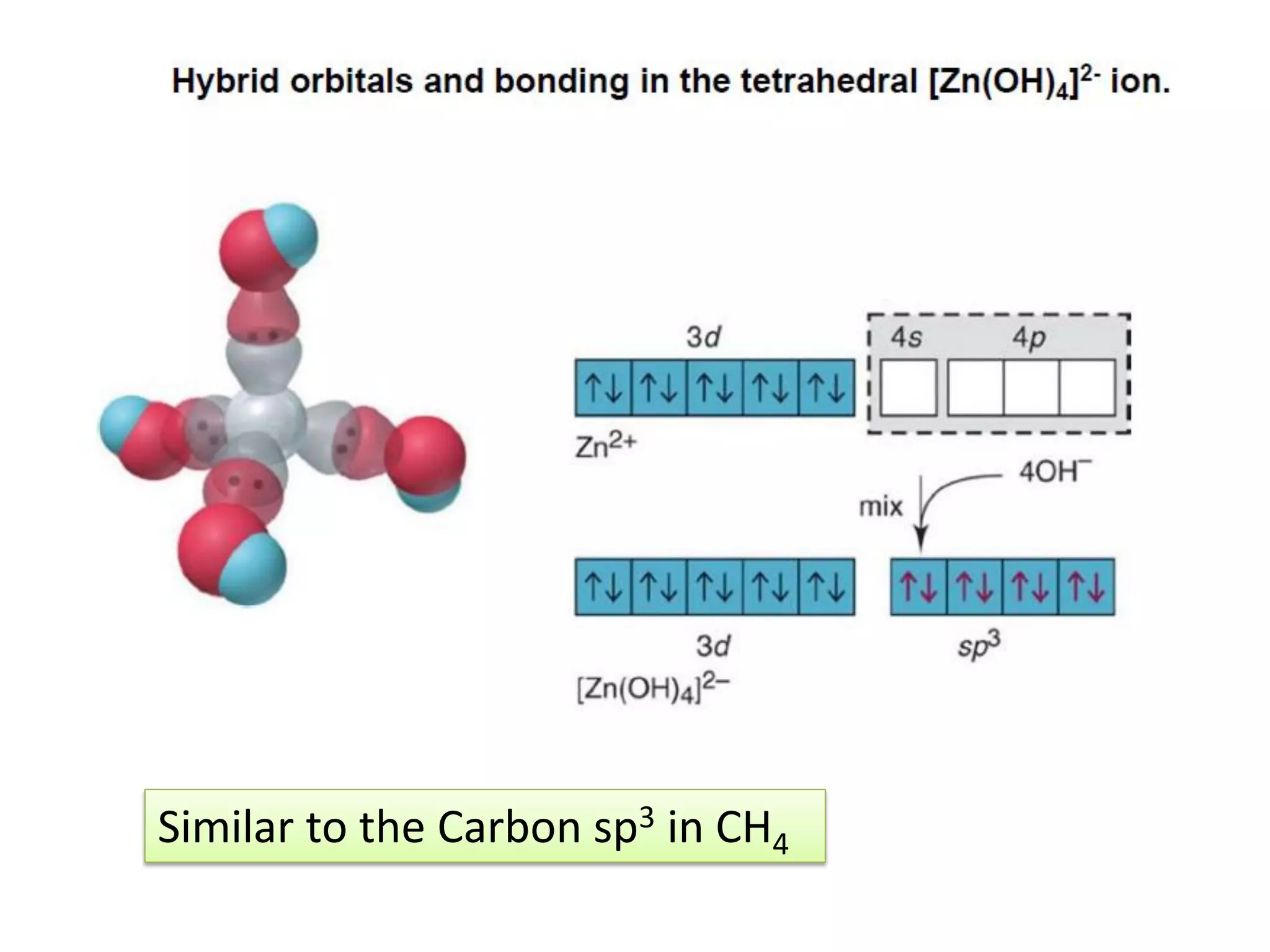
(a) Consider [NiCl4]2+:
Ni0 = 4s2 3d8 Ni2+ = 4s0 3d8
3d 4s 4p
sp3](https://image.slidesharecdn.com/bonding-in-coordination-complexes-i-160213160117/75/Bonding-in-coordination-complexes-Part-1-15-2048.jpg)
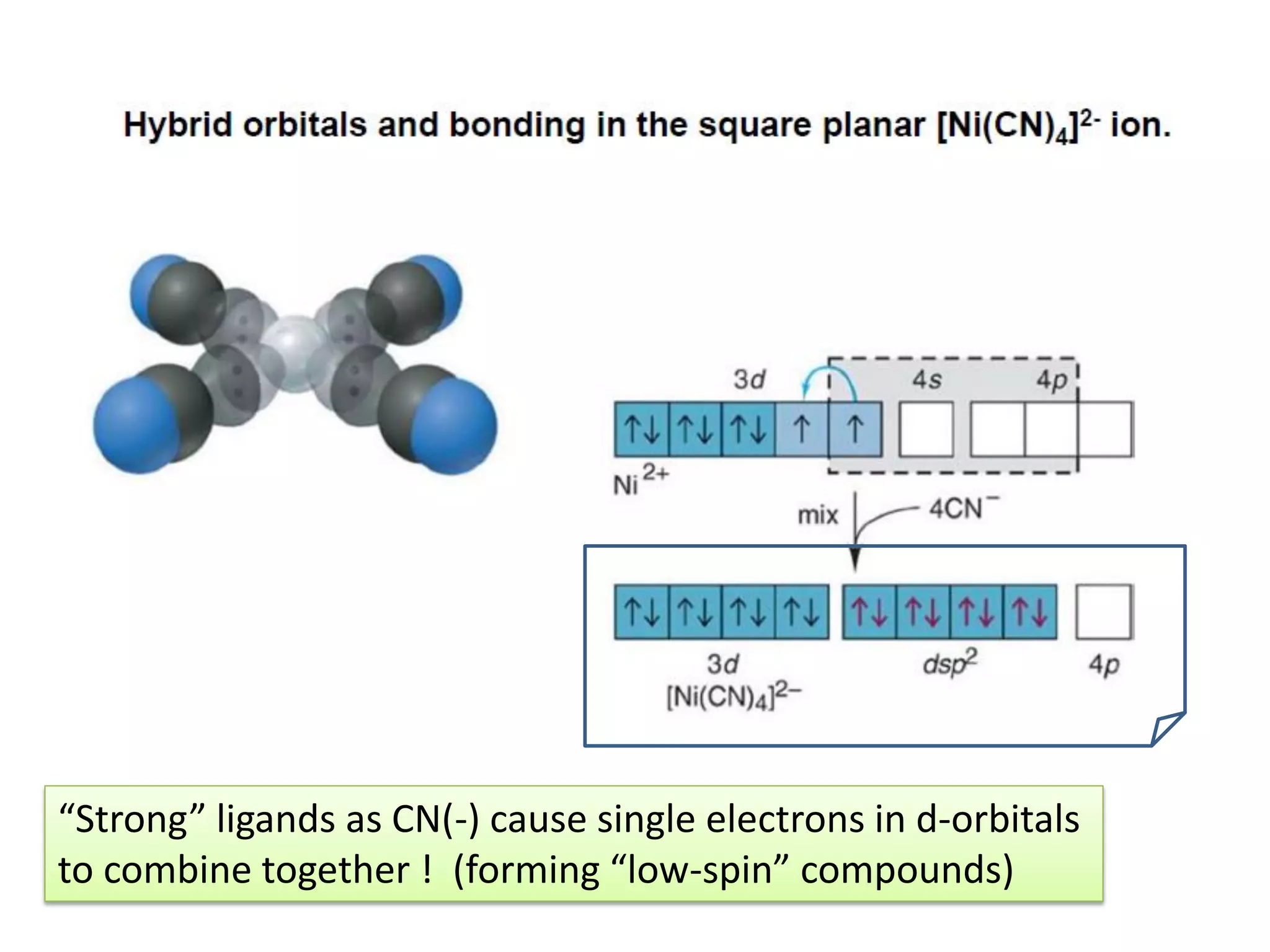
![(b) Consider [Ni(CN)4]2-:
Ni0 = 4s2 3d8 Ni2+ = 4s0 3d8
3d 4s 4p
dsp2 4pz3d
Strong ligands
cause electron
pairing](https://image.slidesharecdn.com/bonding-in-coordination-complexes-i-160213160117/75/Bonding-in-coordination-complexes-Part-1-16-2048.jpg)
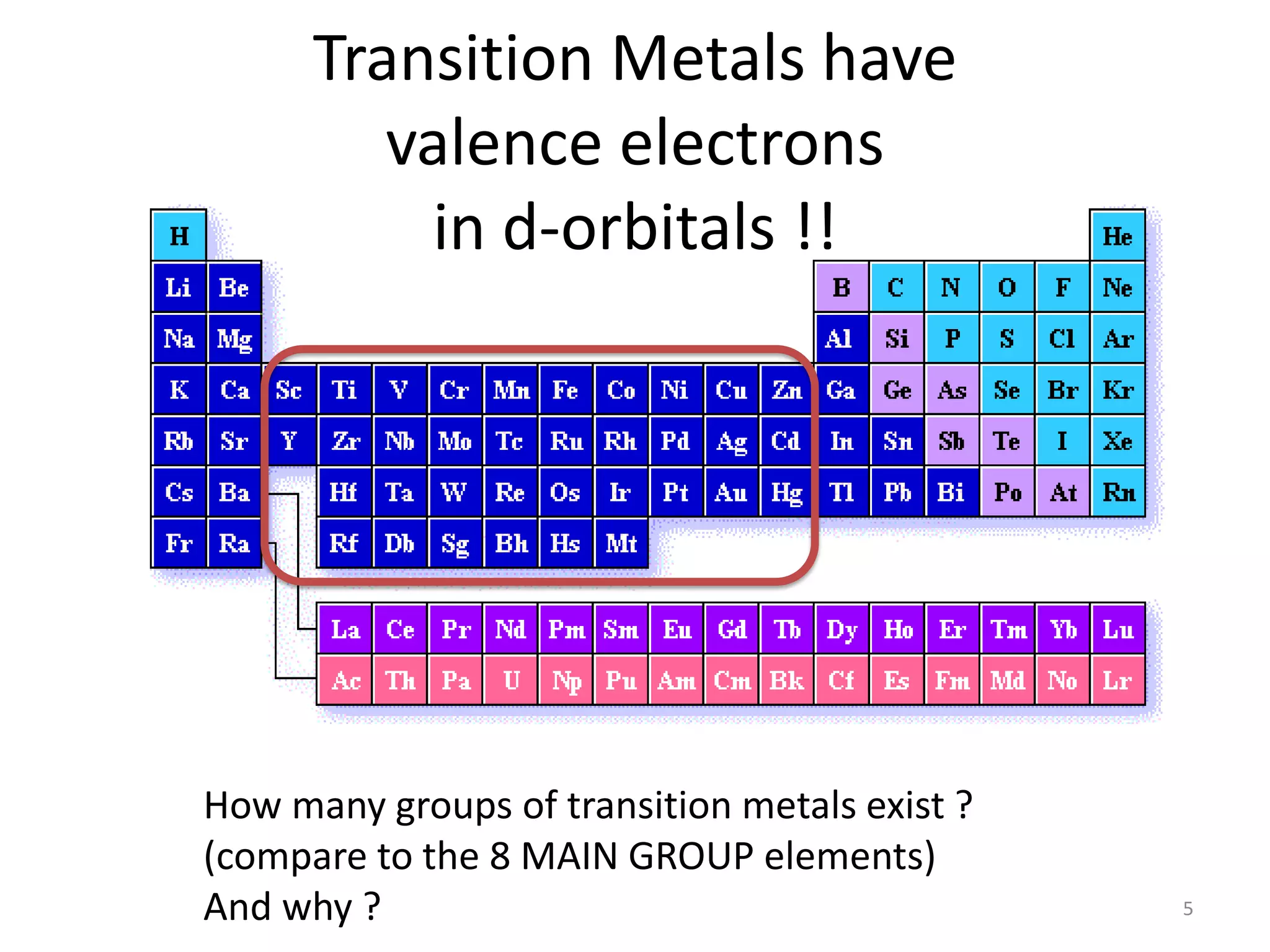
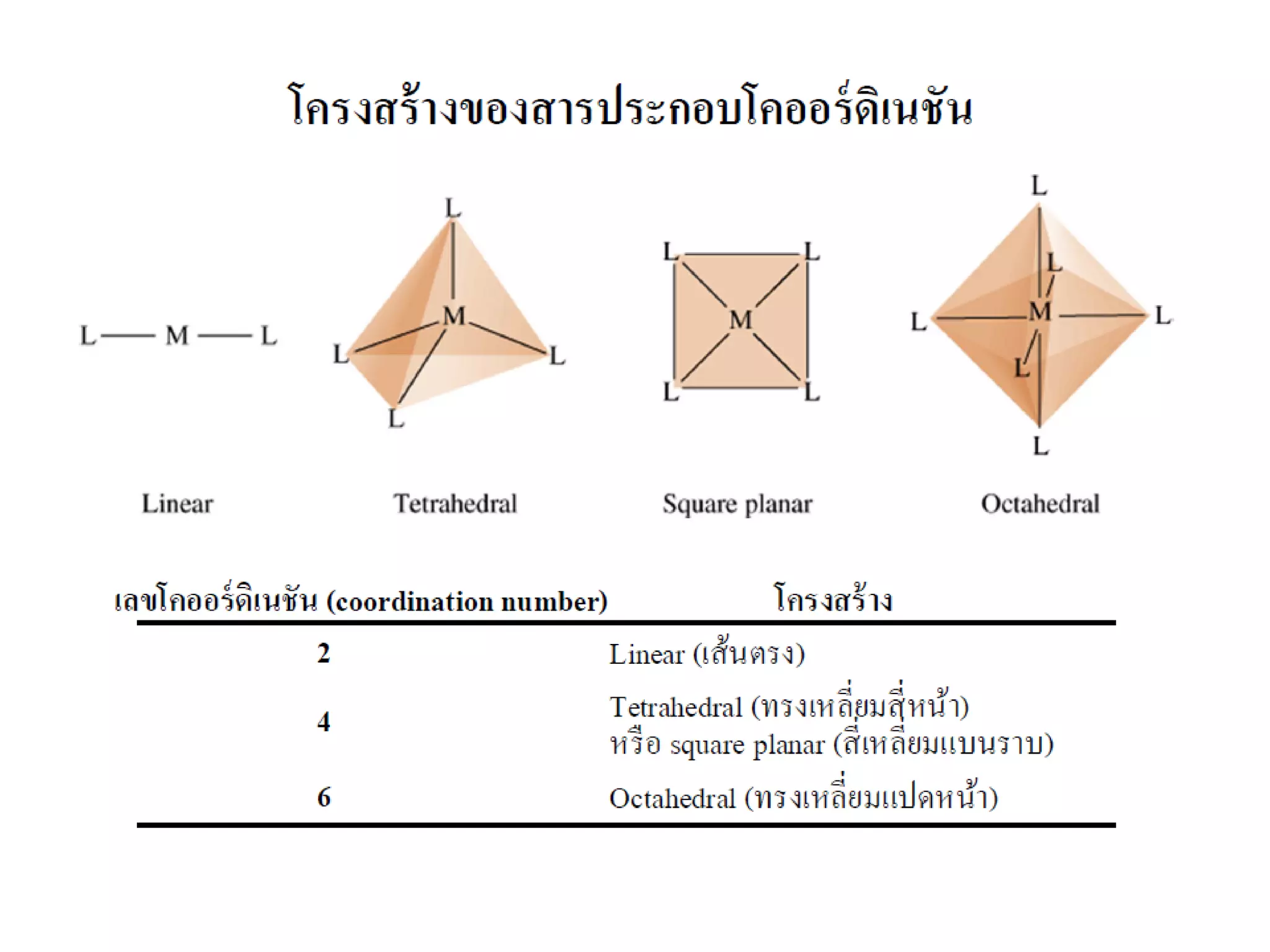
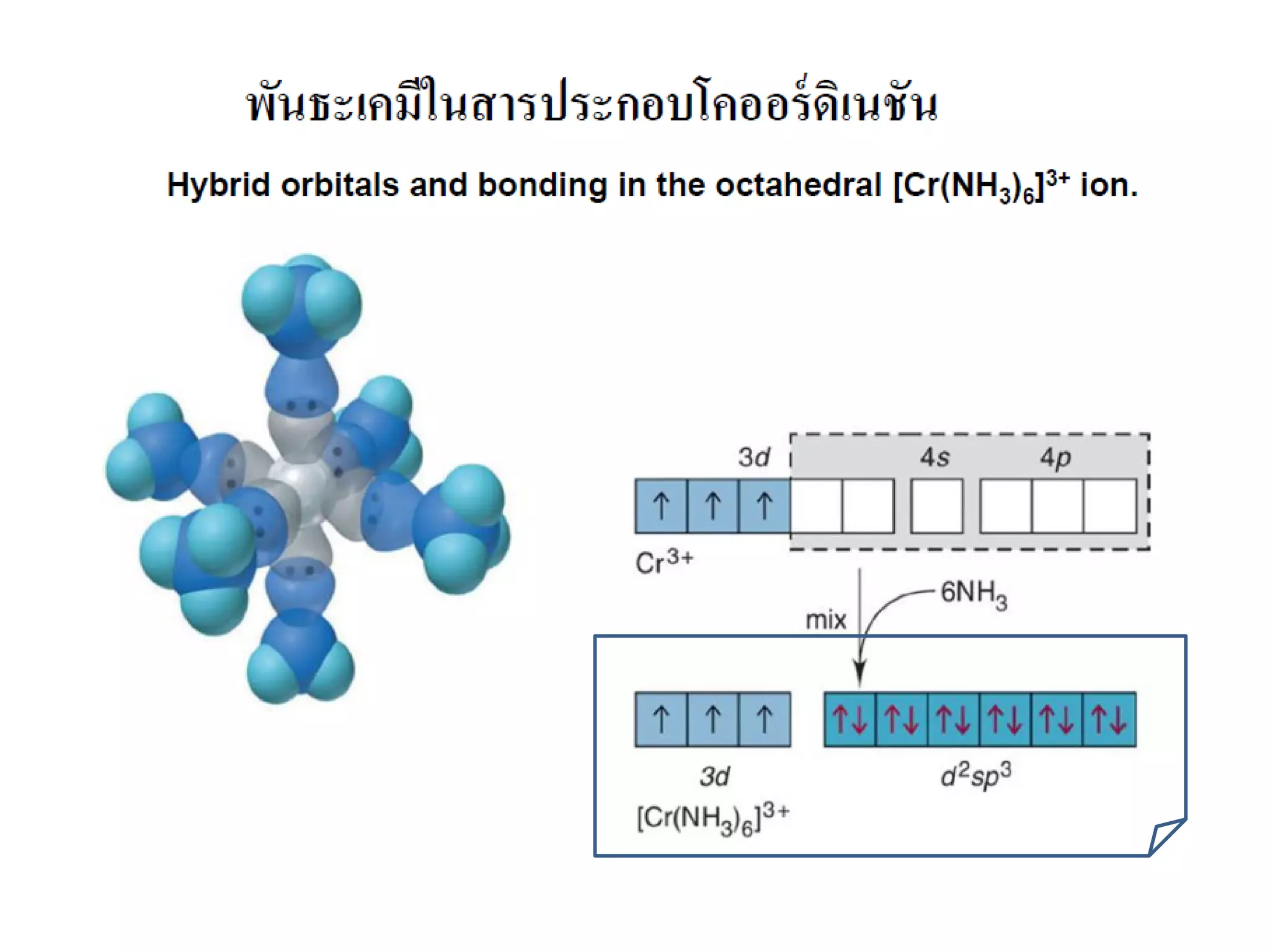
![Octahedral compounds
We need 6 empty hybrid orbitals on the metal
-> for octahedral shape, we need:
(a) d2sp3 (“inner shell”) or (b) sp3d2 (“outer shell”)
Example: [CoF6]3- (weak ligands)
How would [Co(NH3)6]3+ look like ?](https://image.slidesharecdn.com/bonding-in-coordination-complexes-i-160213160117/75/Bonding-in-coordination-complexes-Part-1-17-2048.jpg)