The document discusses metal dinitrogen complexes, focusing on their stability, bonding modes, and differences between dinitrogen (N2) and carbon monoxide (CO) ligands. It details the first synthesized dinitrogen complexes and their structural characteristics, highlighting the importance of back-bonding in their formation. Additionally, it emphasizes the potential applications of these complexes in agriculture and nitrogen fixation research.

![Introduction
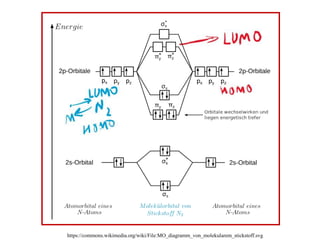
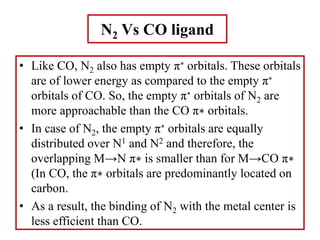
N2 is isoelectronic with NO+ ion and CO ligands.
Dinitrogen metal complexes are not very stable.
Lack of polarity of N2 and less tendency to behave as
a π -acceptor.
[Ru(NH3)5N2]2+ first dinitrogen complex of transition
metal
[Co(PPh3)3H(N2)] first dinitrogen complex of
transition metal prepared from N2.
[Mo(PPh2CH2CH2PPh2)2(N2)2] first dinitrogen
complex of molybdenum](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-2-320.jpg)
![Preparation
The first dinitrogen complex (Pentaamine dinitogen ruthenium
(II) cation) [Ru(NH3)5(N2)]2+ was prepared by the reduction of
commercial ruthenium trichloride by hydrazine in aqueous
solution (1965).
RuCl3 + 4N2H4 → [Ru(NH3)5N2]2+ + ...
Direct reaction of N2
[Co(N2)H(PPh3)3], [RuH2(N2) (PPh3)3] and [FeH2(N2)(PR3)3] (R3
= EtPh2, n-Bu) react direct with nitrogen to form dinitrogen
complex.
[MH2(PR3)3] + N2 → [MHn-2(N2)(PR3)3 + H2](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-3-320.jpg)
![ N2 ligand can be easily displaced by water and formation of
dinuclear dinitrogen complex can be formed.
[Ru(NH3)5N2]2+ + [Ru(NH3)5H2O]2+ [Ru(NH3)5N2(NH3)5Ru]4+
+ H2O
From nitrous oxide
[Ru(NH3)5Cl]Cl2 + Zn/Hg [Ru(NH3)5H2O]2+
[Ru(NH3)5H2O]2+ + N2O [Ru(NH3)5NNO]2+
[Ru(NH3)5NNO]2+ + Zn/Hg [Ru(NH3)5N2]2+
Preparation](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-4-320.jpg)
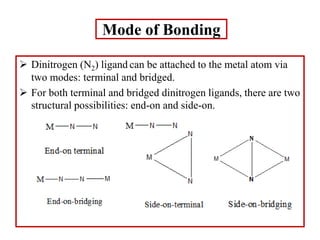


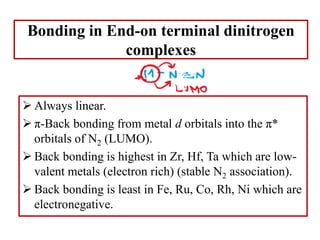
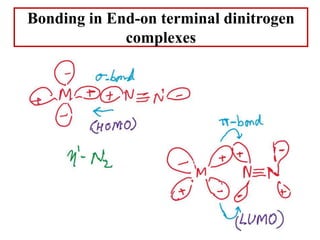
![Terminal end-on
Terminal end-on (one end-on N2 ligand)
N2 is sigma-donor and pi-accepter.
[Ru(NH3)5N2]2+
[IrCl(N2)(PPh3)2]
Terminal end-on (more than one end-on N2 ligand)
mer-[Mo(N2)3(PPrn
2Ph)3]
[Mo(N2)2(Ph2PCH2CH2PPh2)2]](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-14-320.jpg)
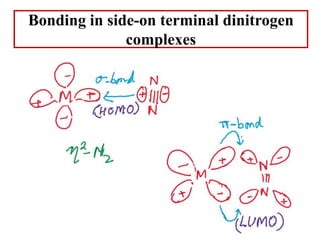
![Terminal side-on
one side N2 ligand
High energy compounds
Less stable
N2 is pi donor
[Os(NH3)5(η2-N2)]2+ (metastable)](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-15-320.jpg)
![Bridging end-on
Multinuclear dinitrogen complexes
{[Ru(NH3)5]2(μ-N2)}4+
Iron dinitrogen complexes
Fe-N-N-Fe](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-16-320.jpg)
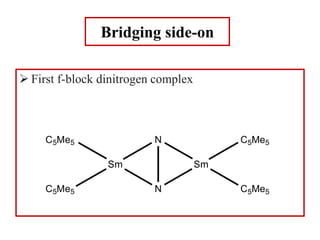
![Both terminal and bridging nitrogen
[(η1-N2)(η5-C5Me5)2Zr]2(μ2, η2-N2)](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-17-320.jpg)
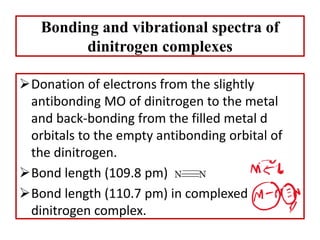
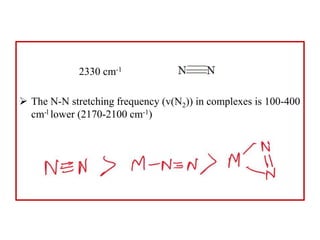
![Uses of dinitrogen complexes
Dinitrogen complexes may be used in agriculture and
industries and are of great interest, especially as possible
intermediates to study the reactions that may simulate natural
processes of nitrogen fixation.
The discovery of dinitrogen complexes is helpful in
investigating the possibility of nitrogen fixation via such
complexes.
Mo(N2)2(dpe)2 + 6H+ → 2NH3 + N2 + Mo (VI) products
where dpe = Ph2PCH2CH2PPh2[1,2- bis(diphenyl phosphine
ethane]](https://image.slidesharecdn.com/dinitrogencomplexes-210502141453/85/Dinitrogen-complexes-21-320.jpg)
