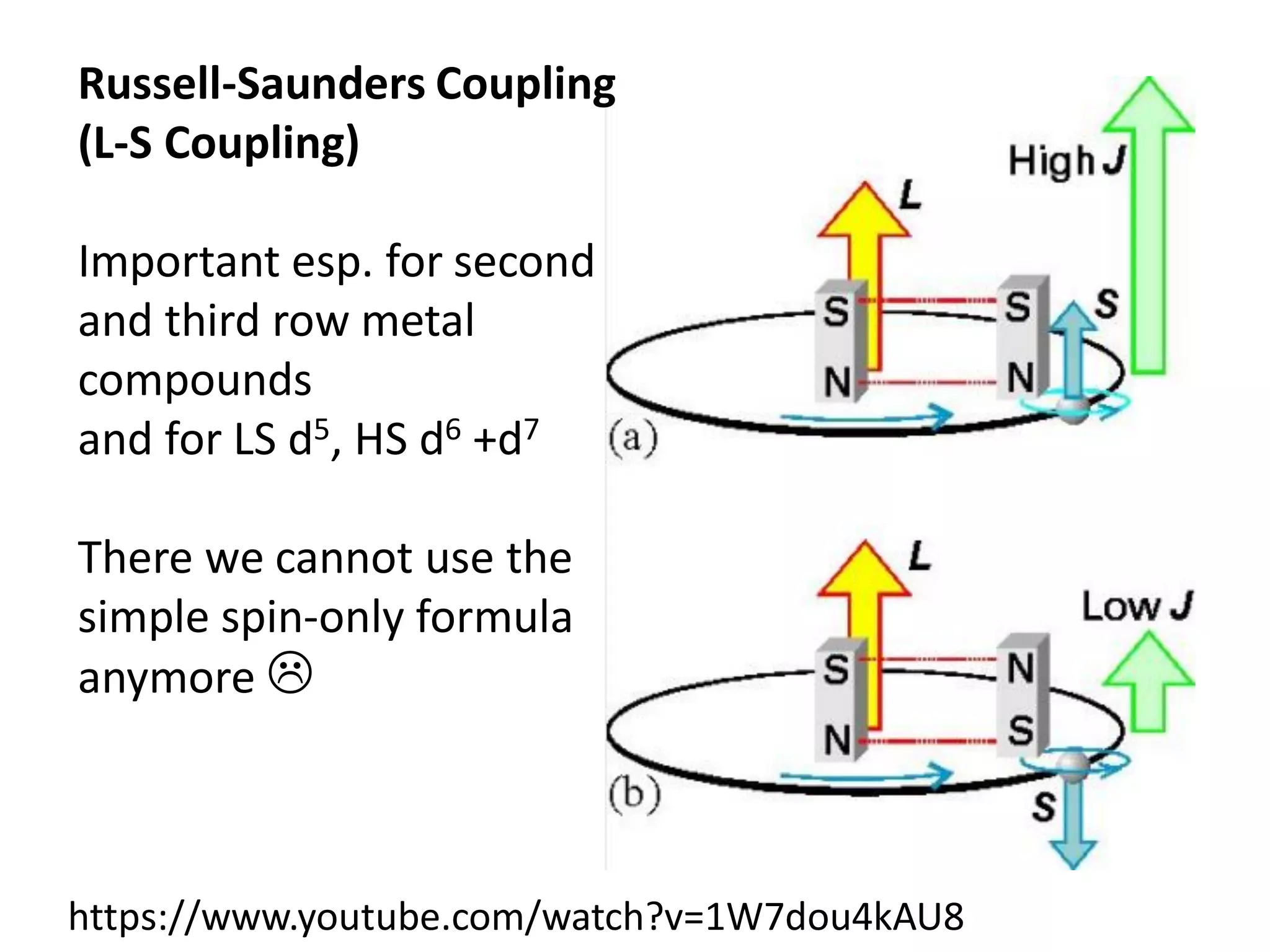
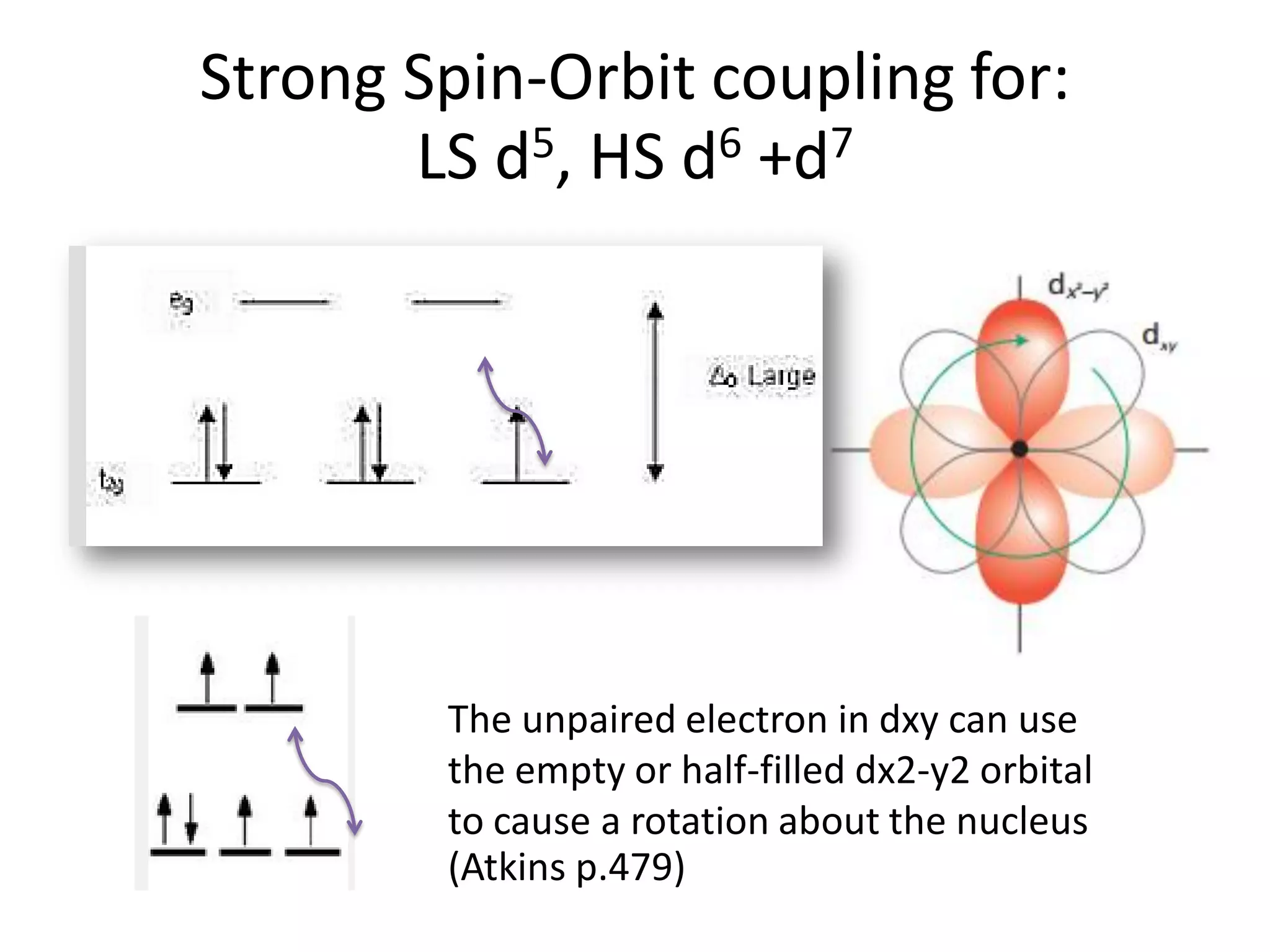
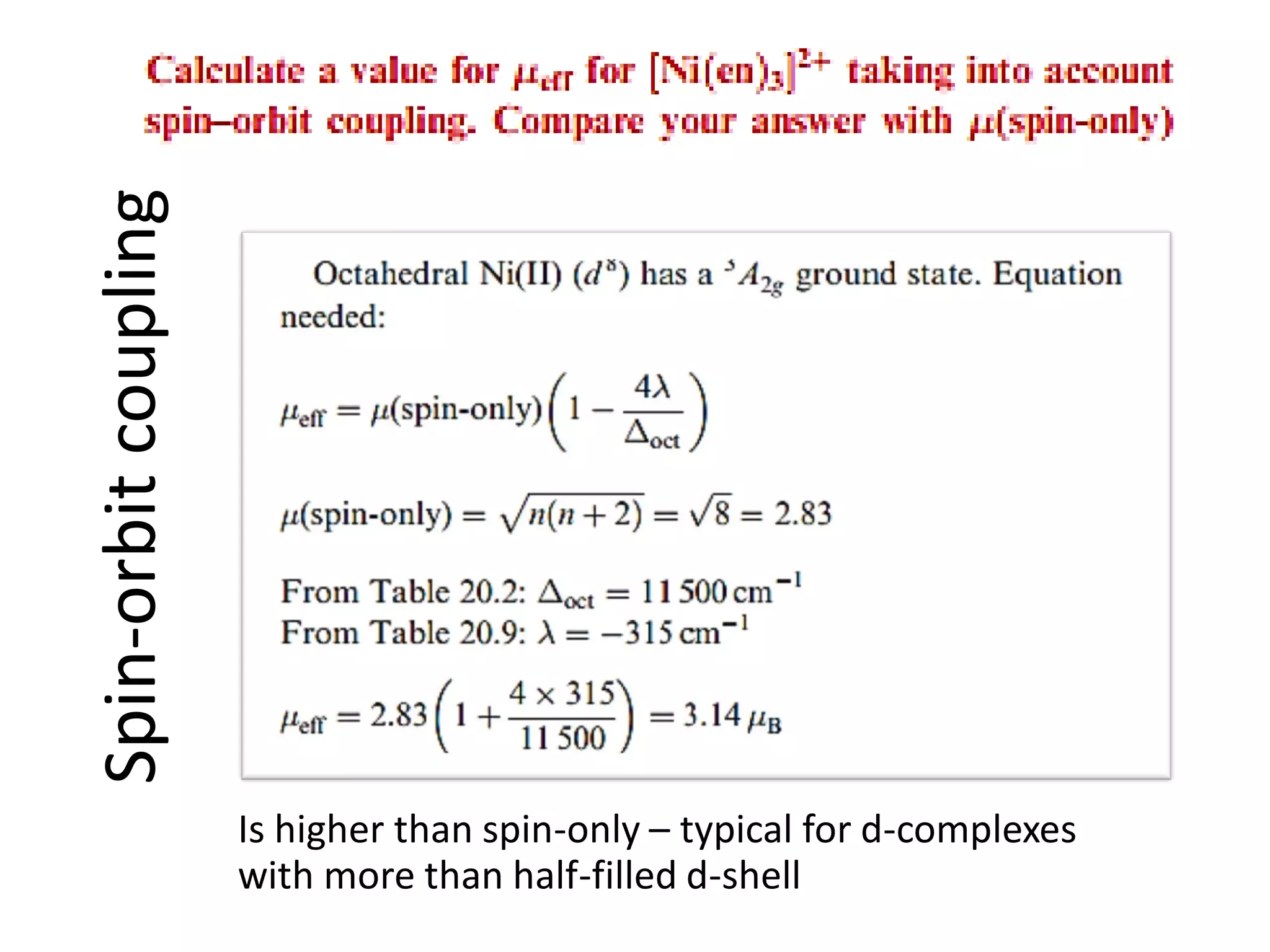
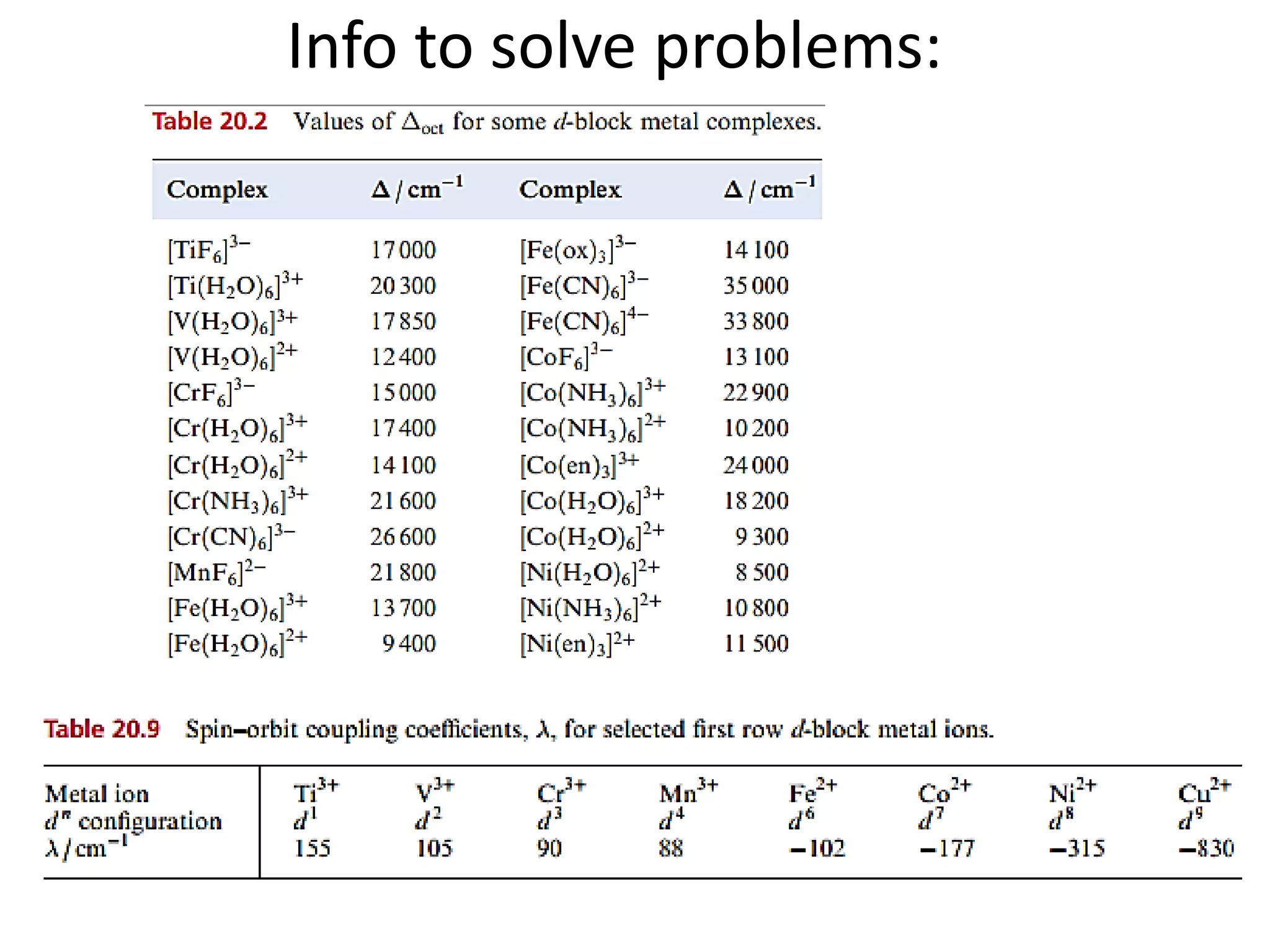
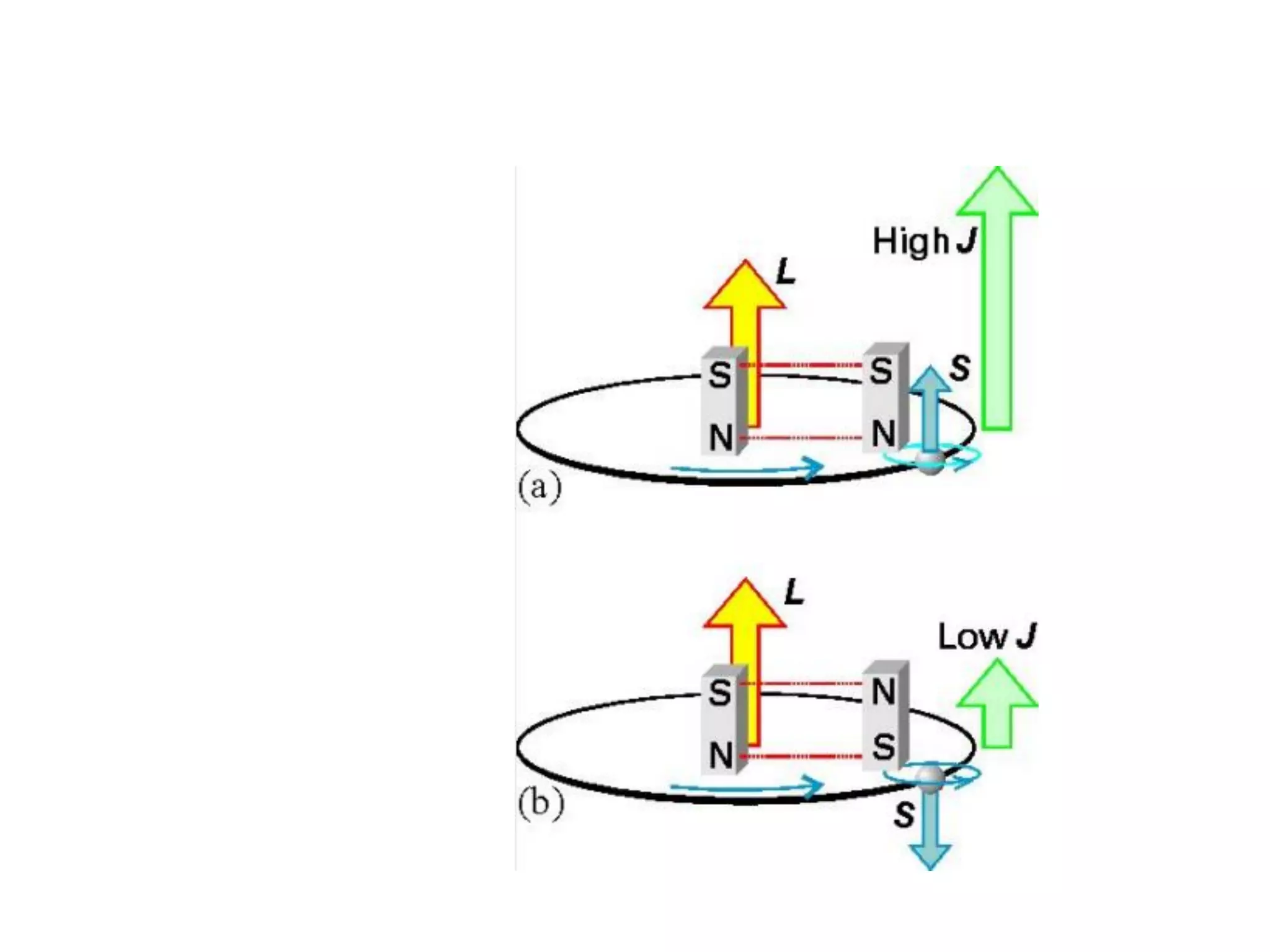
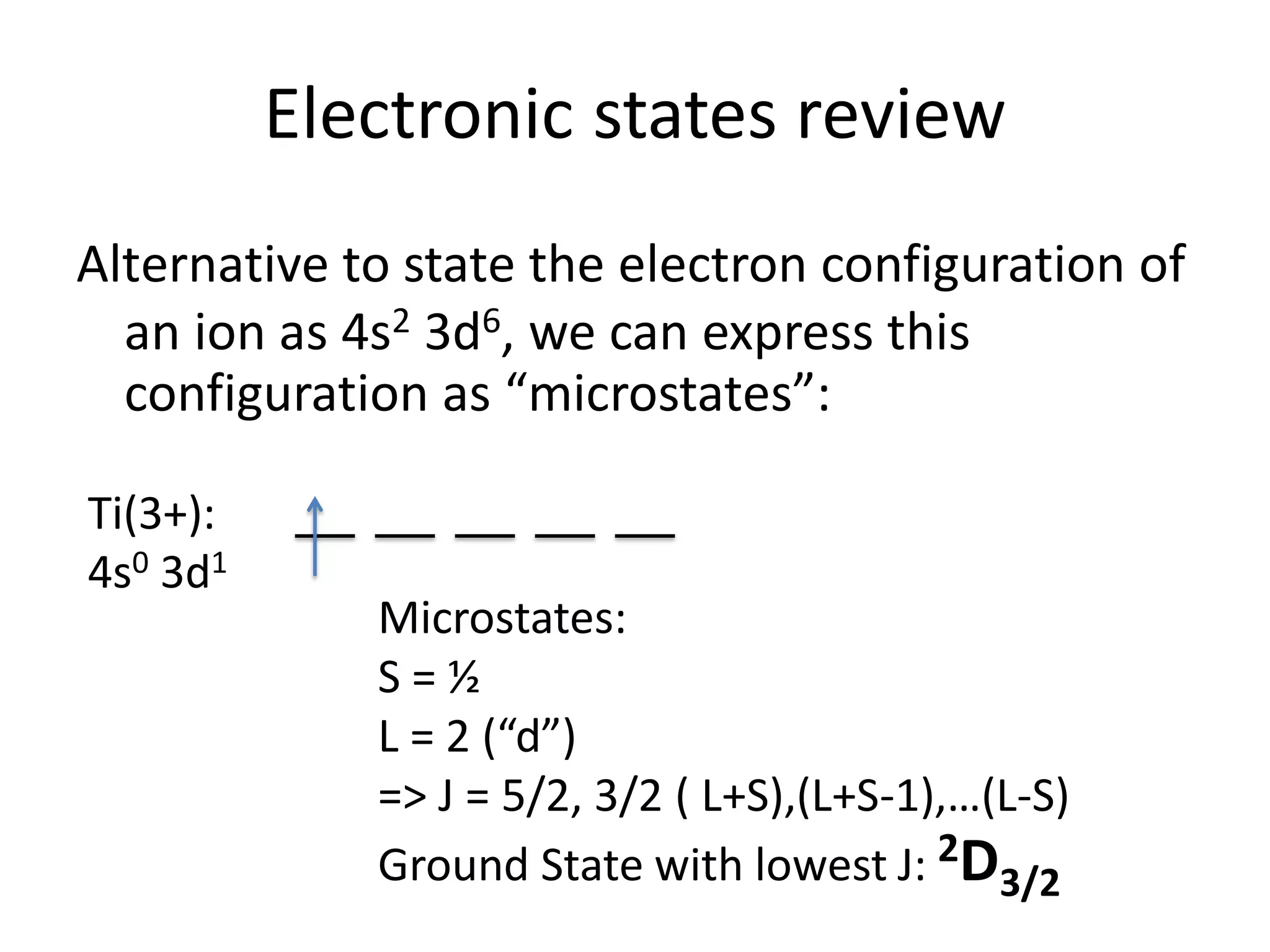
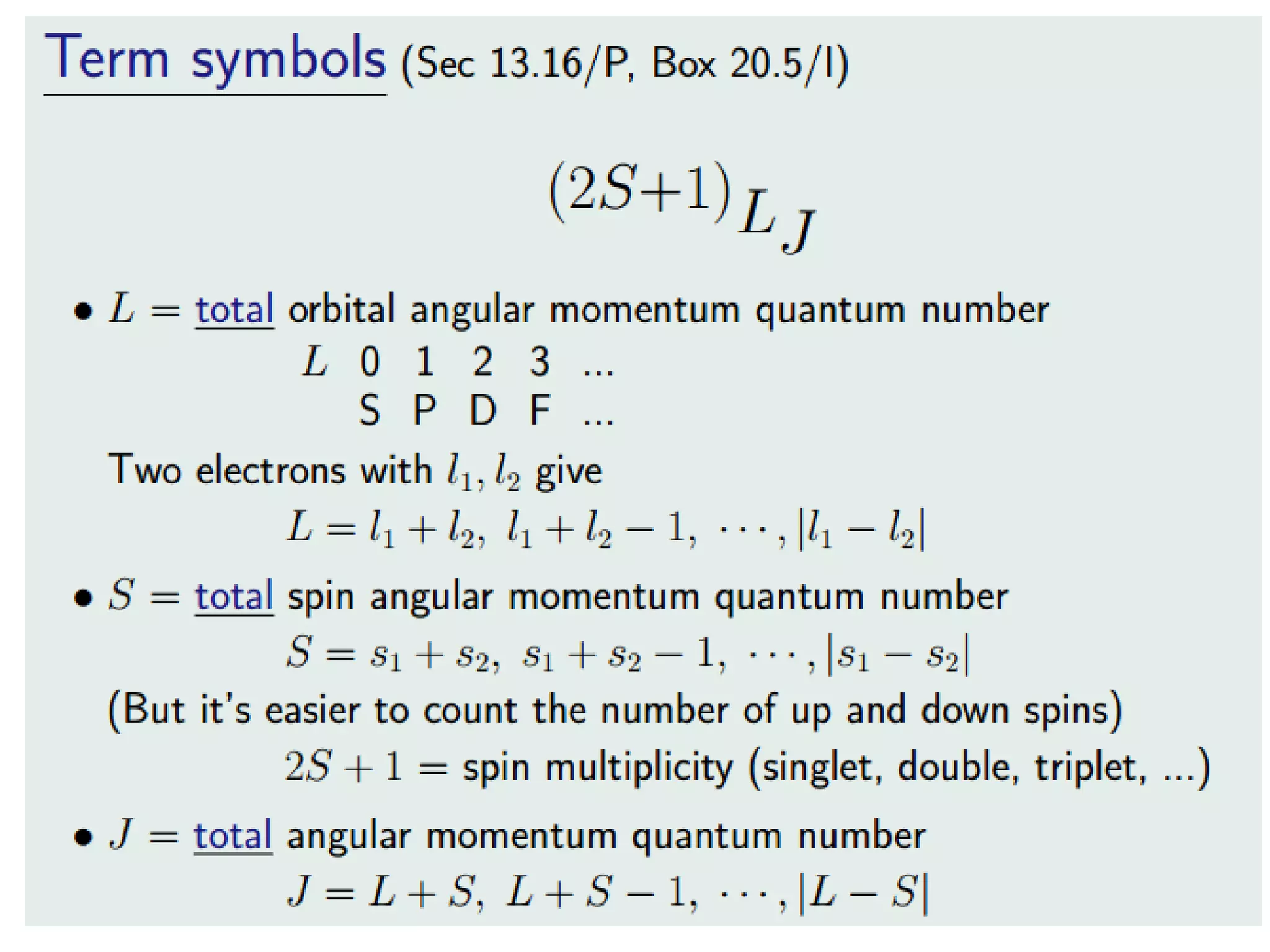
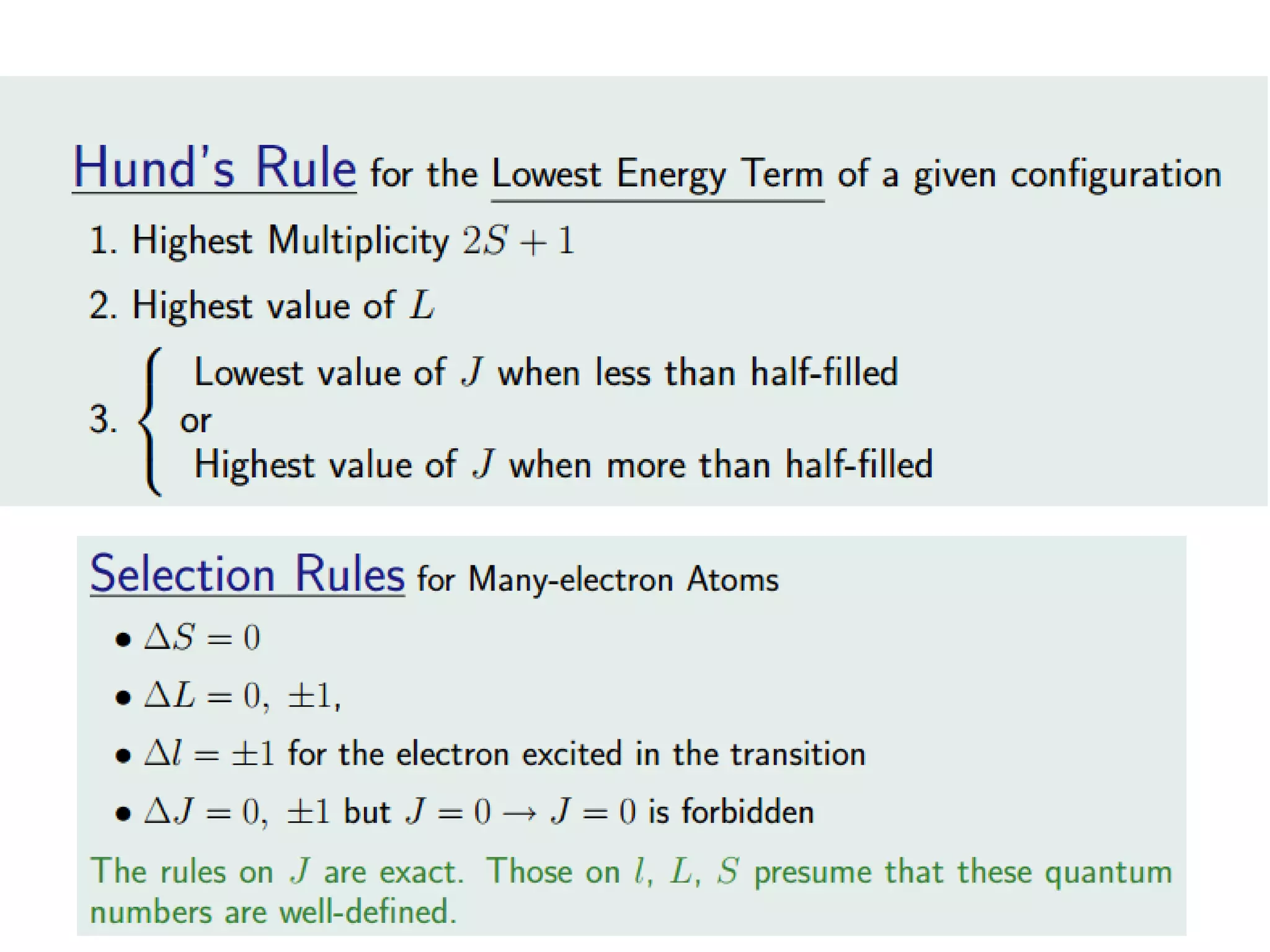
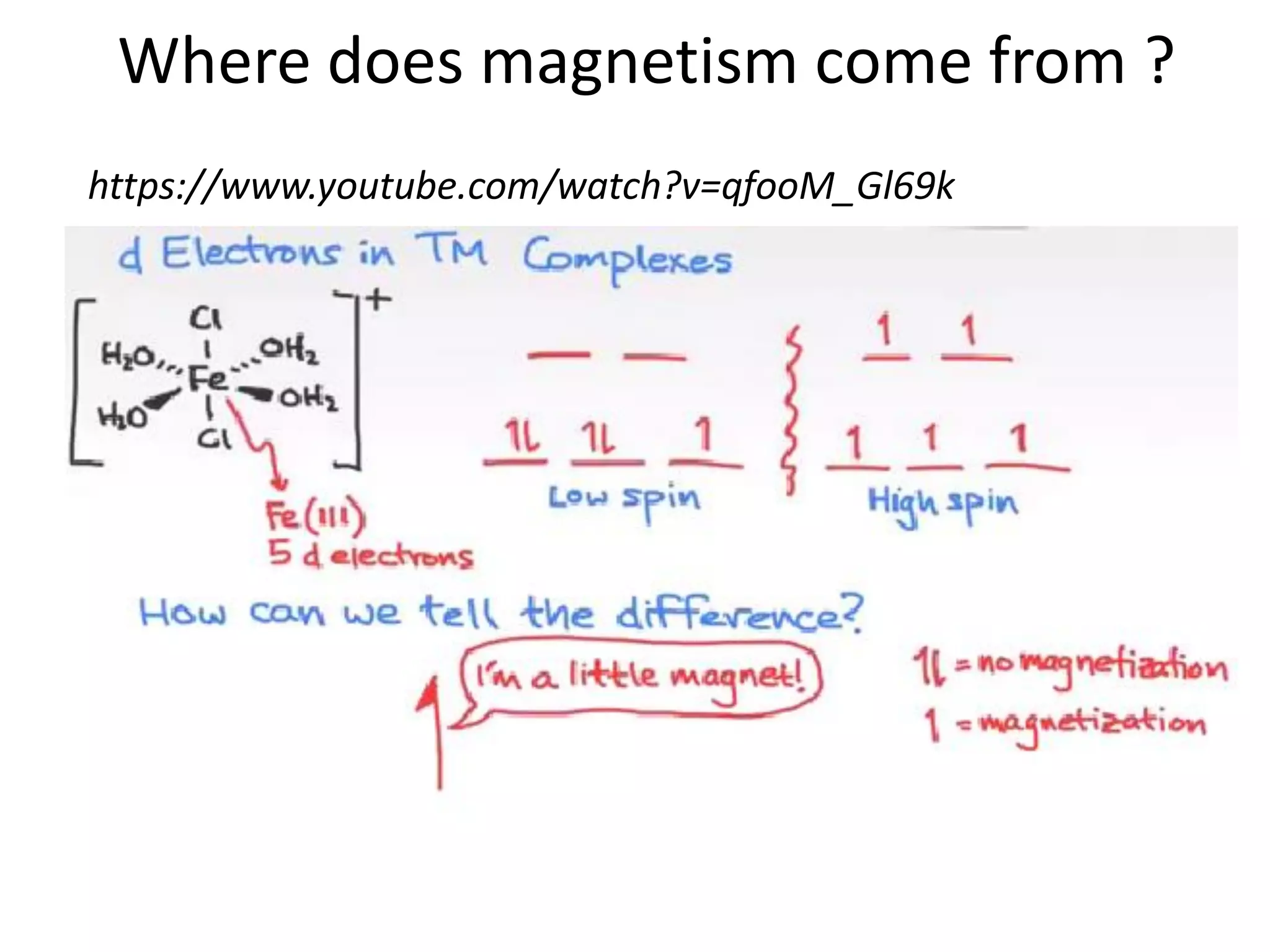
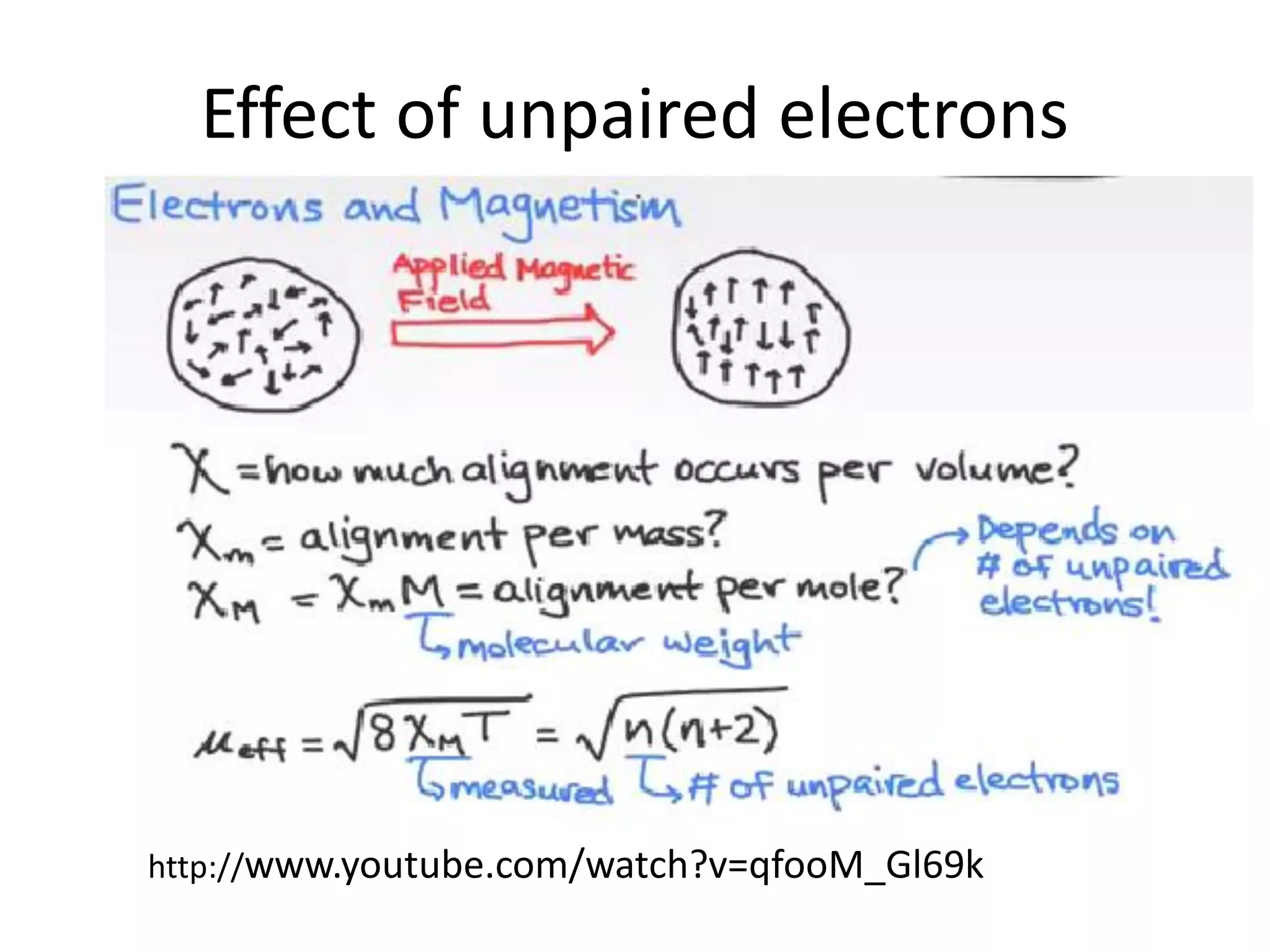
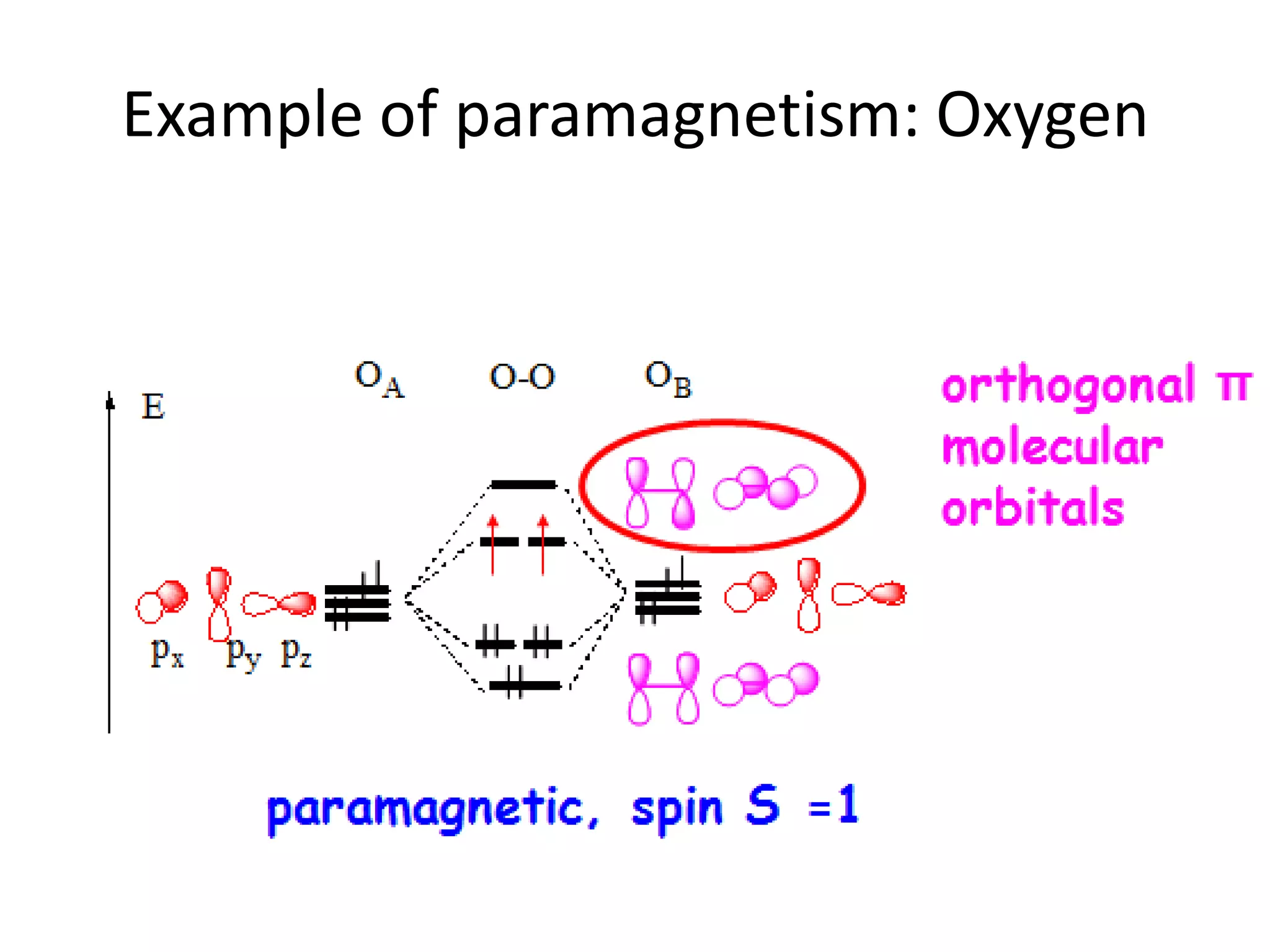
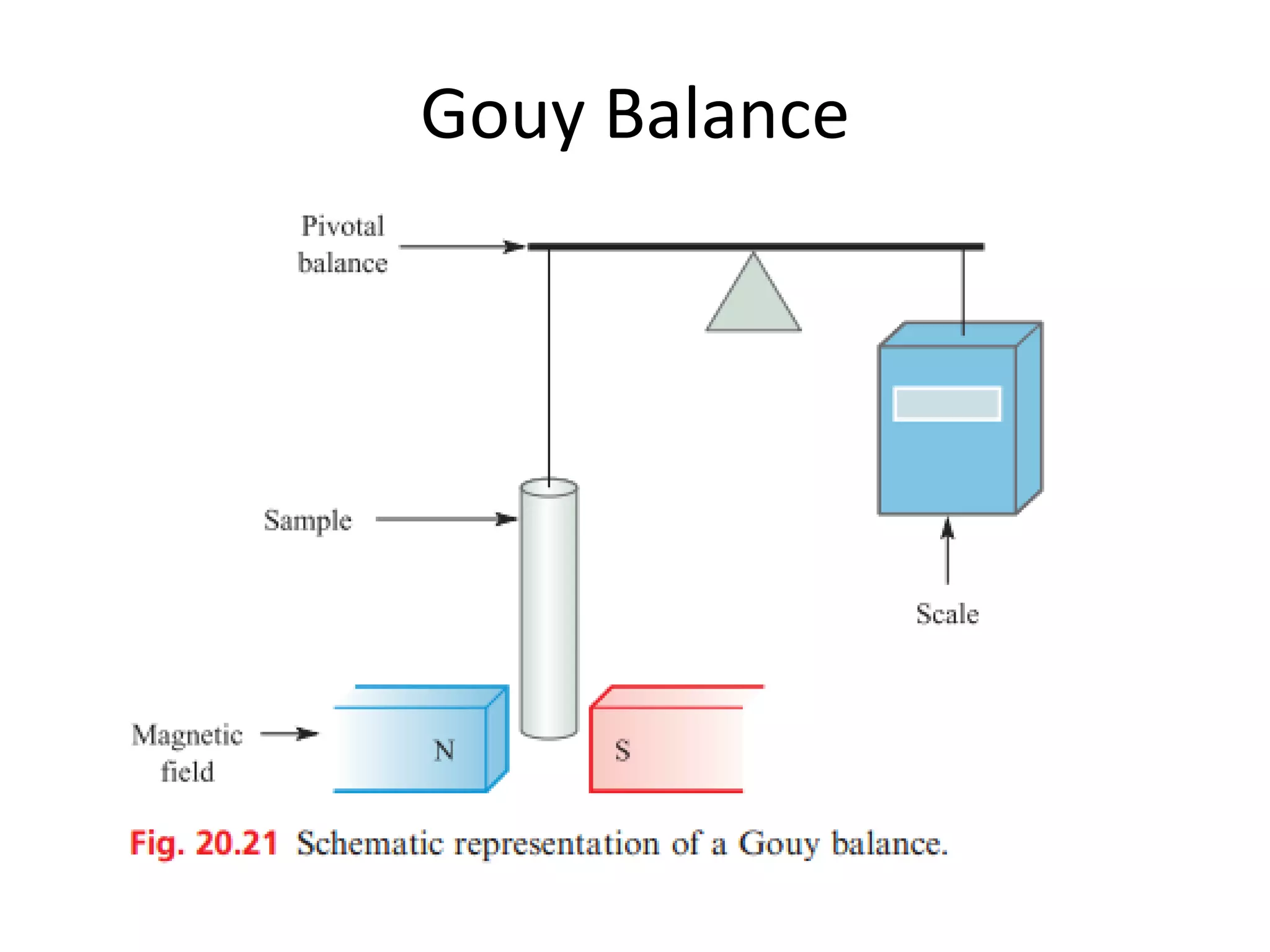
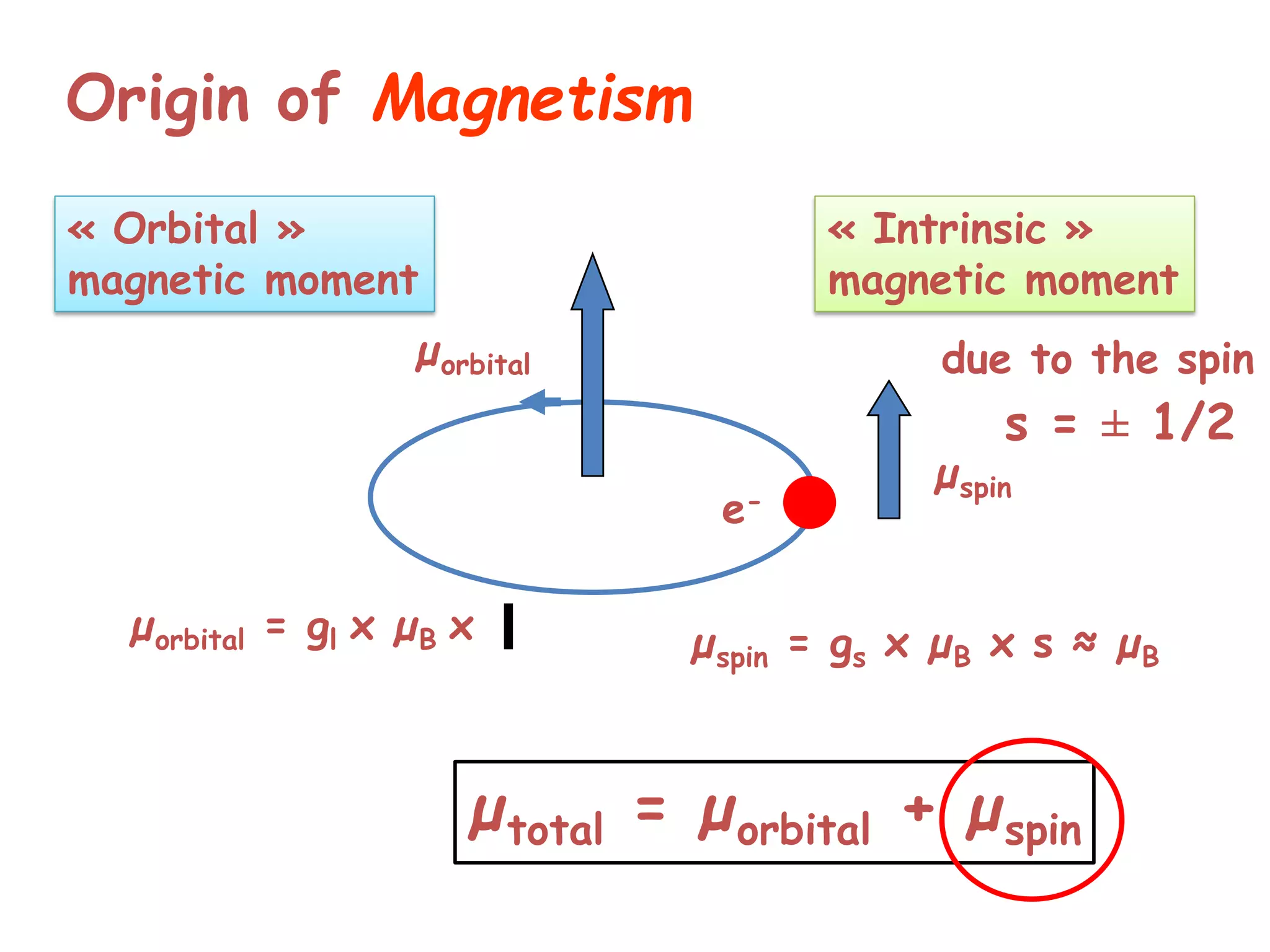
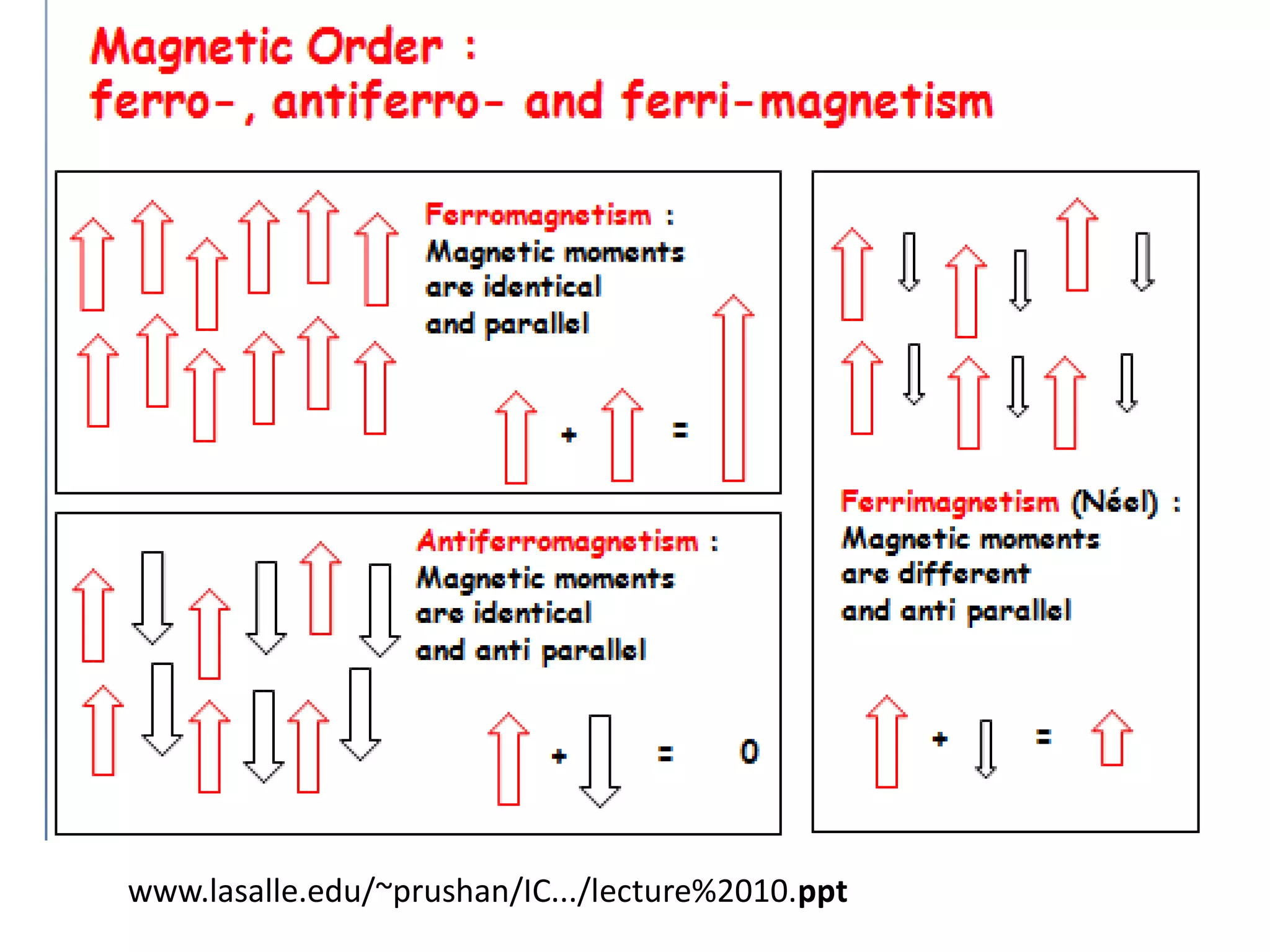
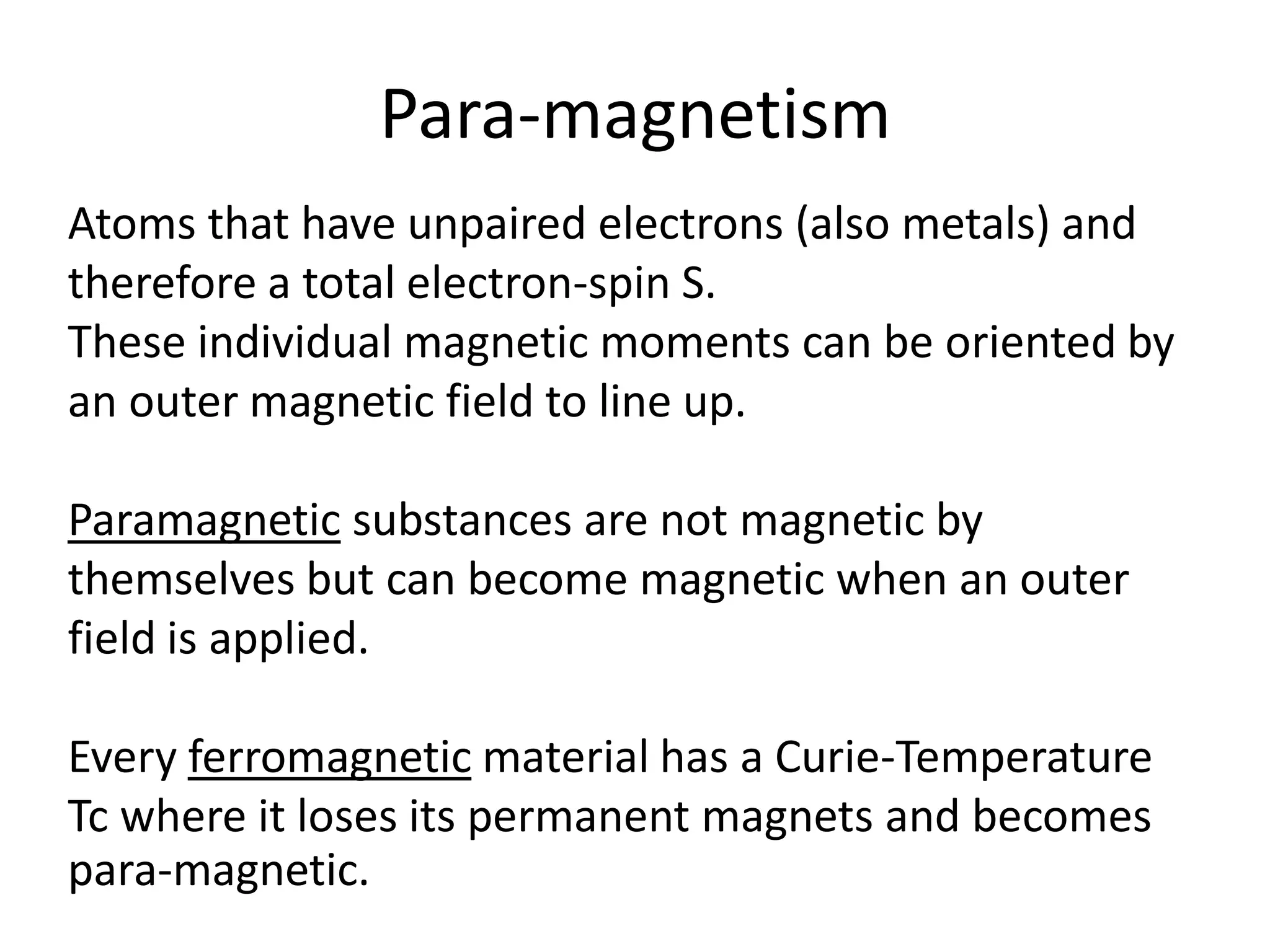
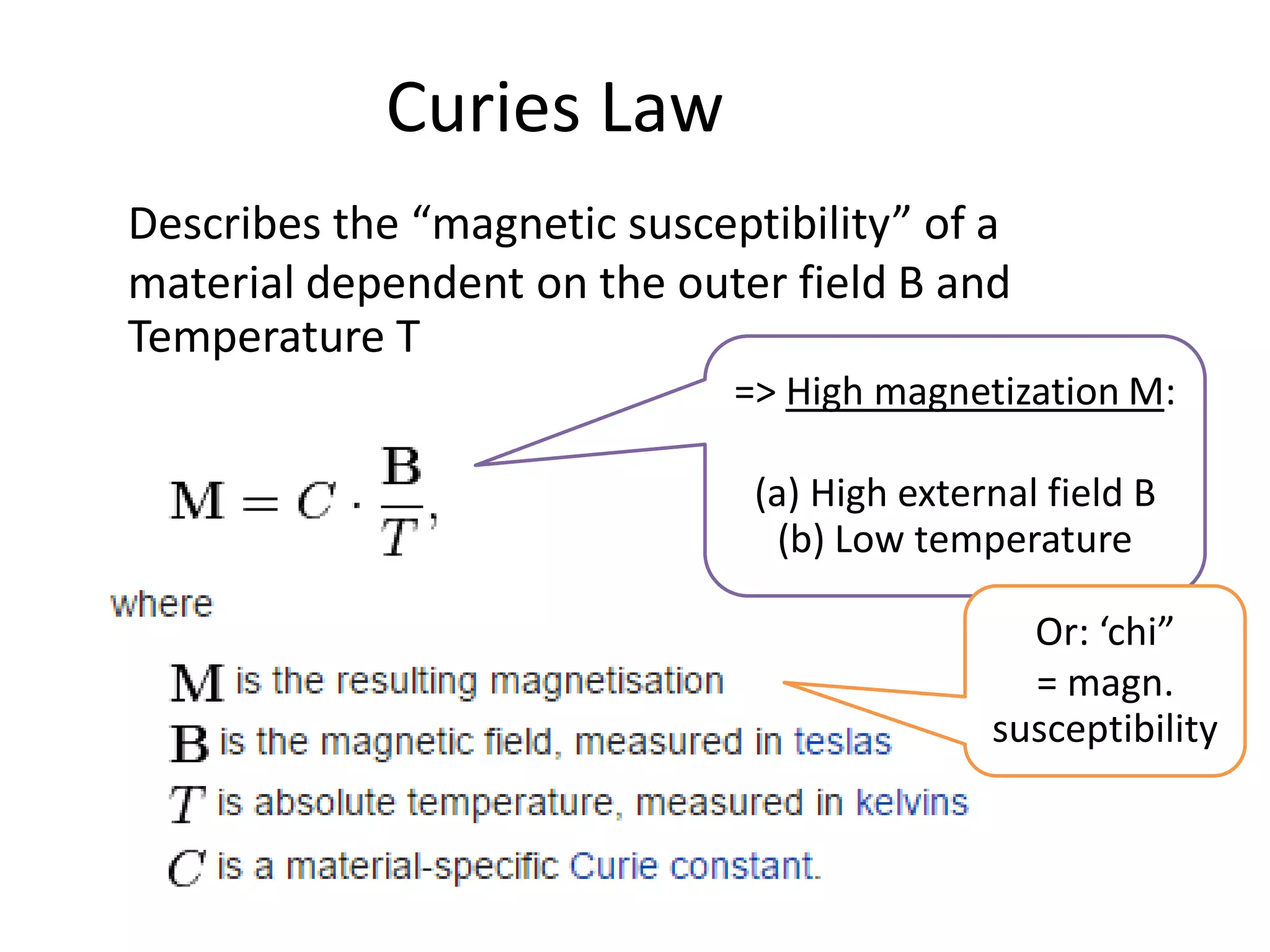
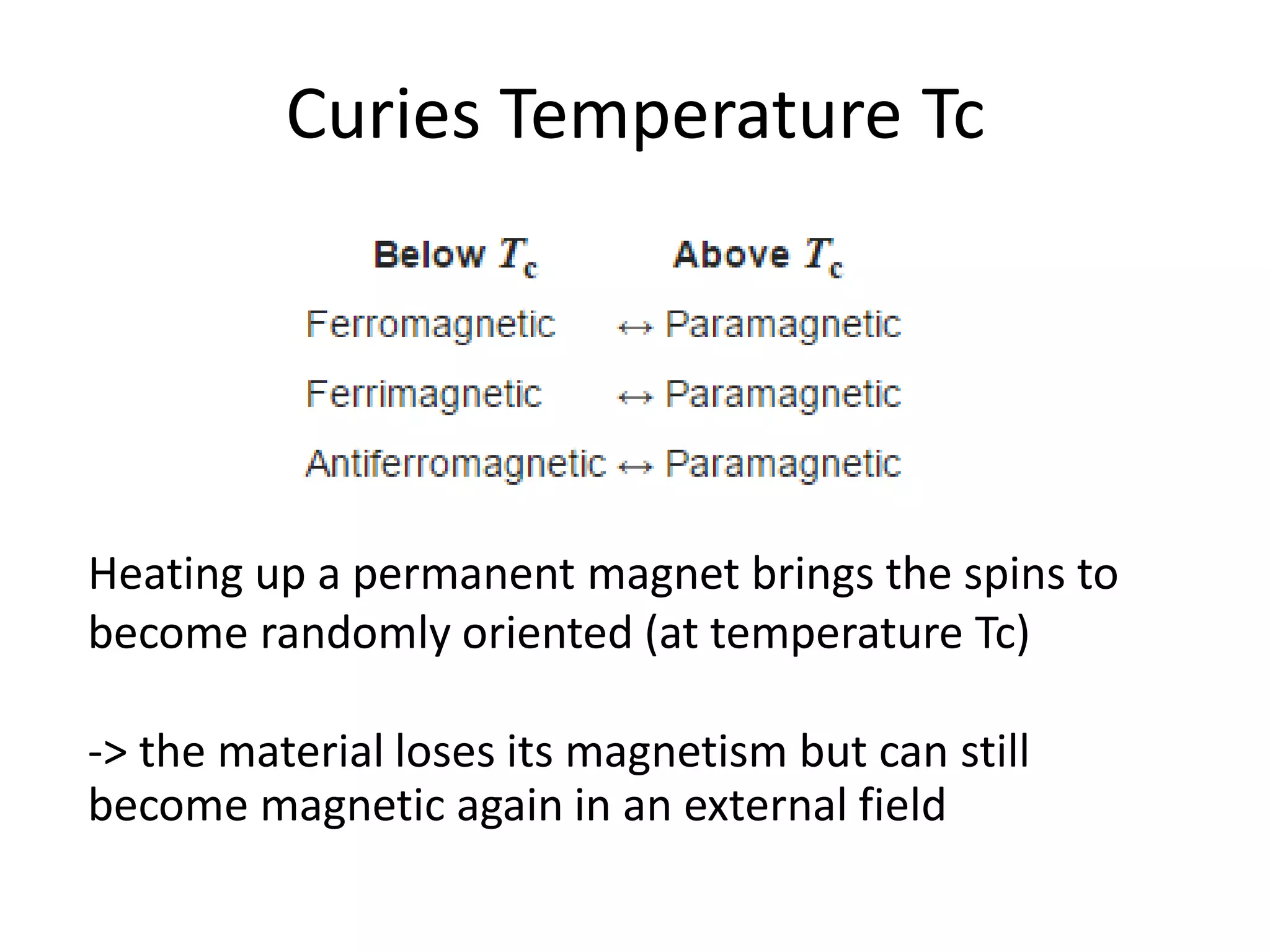
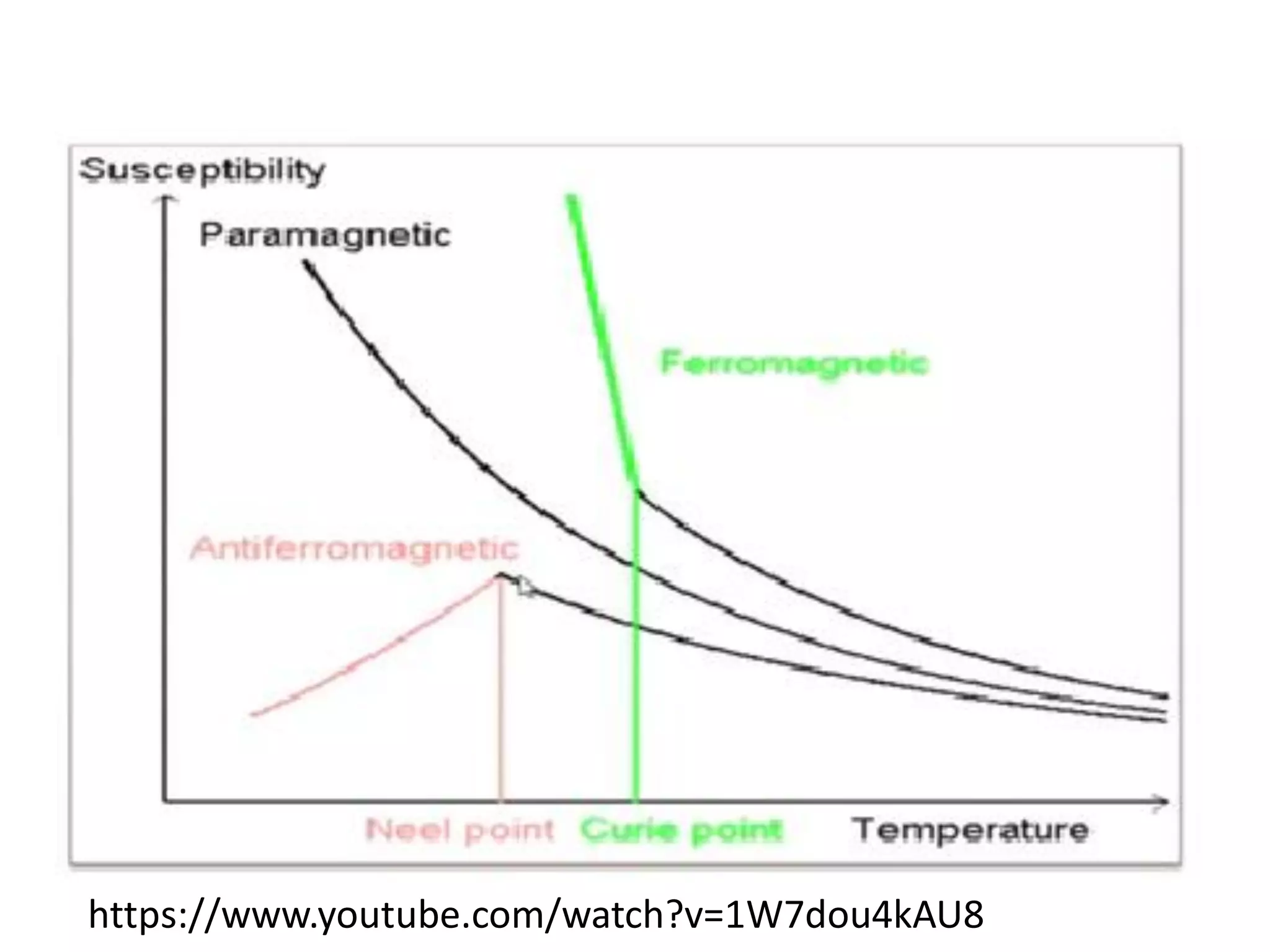
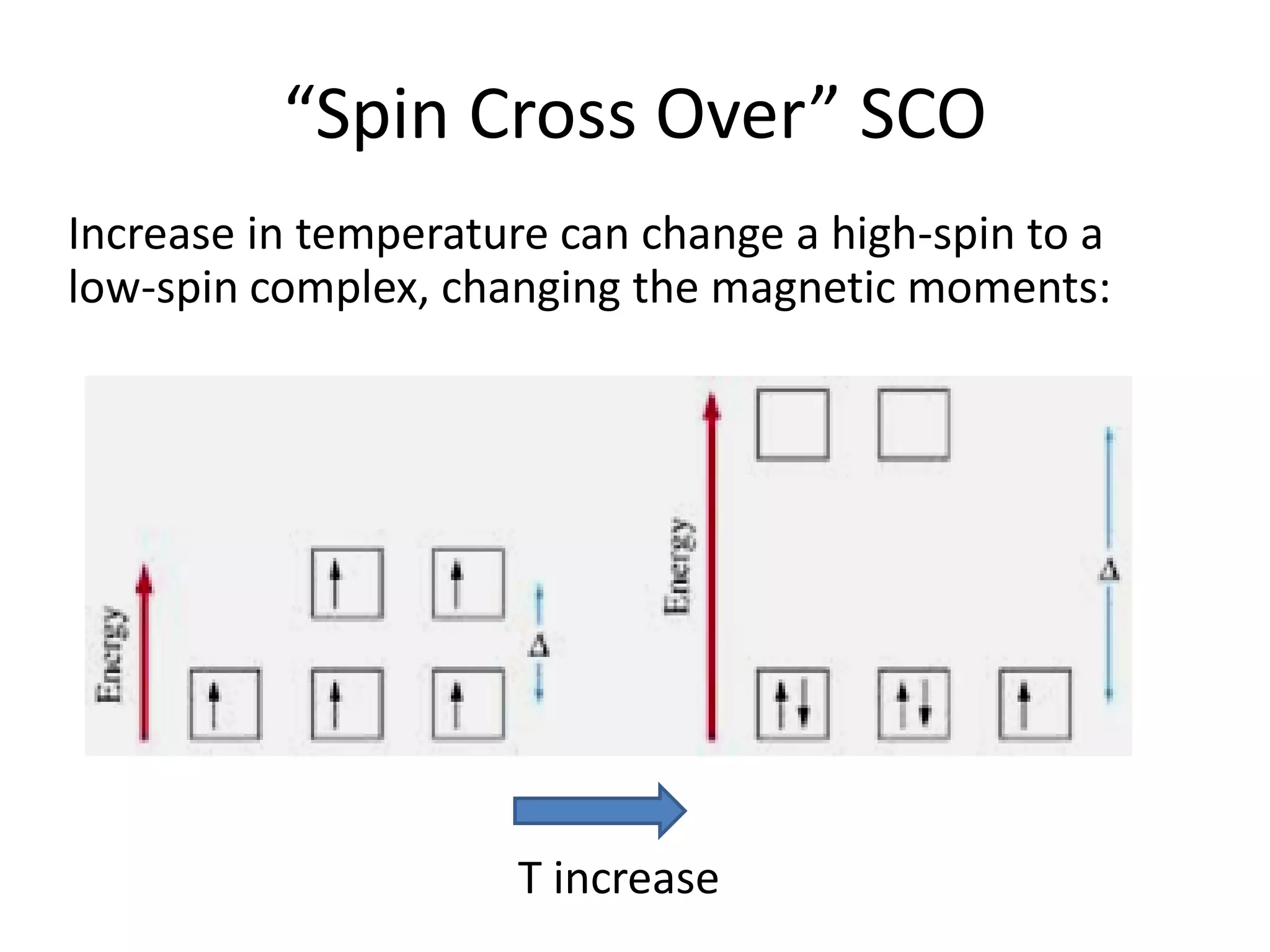
Paramagnetism arises from unpaired electrons in coordination compounds. Each unpaired electron contributes a magnetic moment due to both its spin and orbital angular momentum. When an external magnetic field is applied, the magnetic moments of paramagnetic compounds can align, causing the compound to become temporarily magnetic. The magnetic susceptibility and magnetization of paramagnetic compounds increases with applied field strength and decreases with increasing temperature according to Curie's law. Some transition metal complexes can switch between low-spin and high-spin electronic configurations in response to temperature changes, altering their magnetic properties.


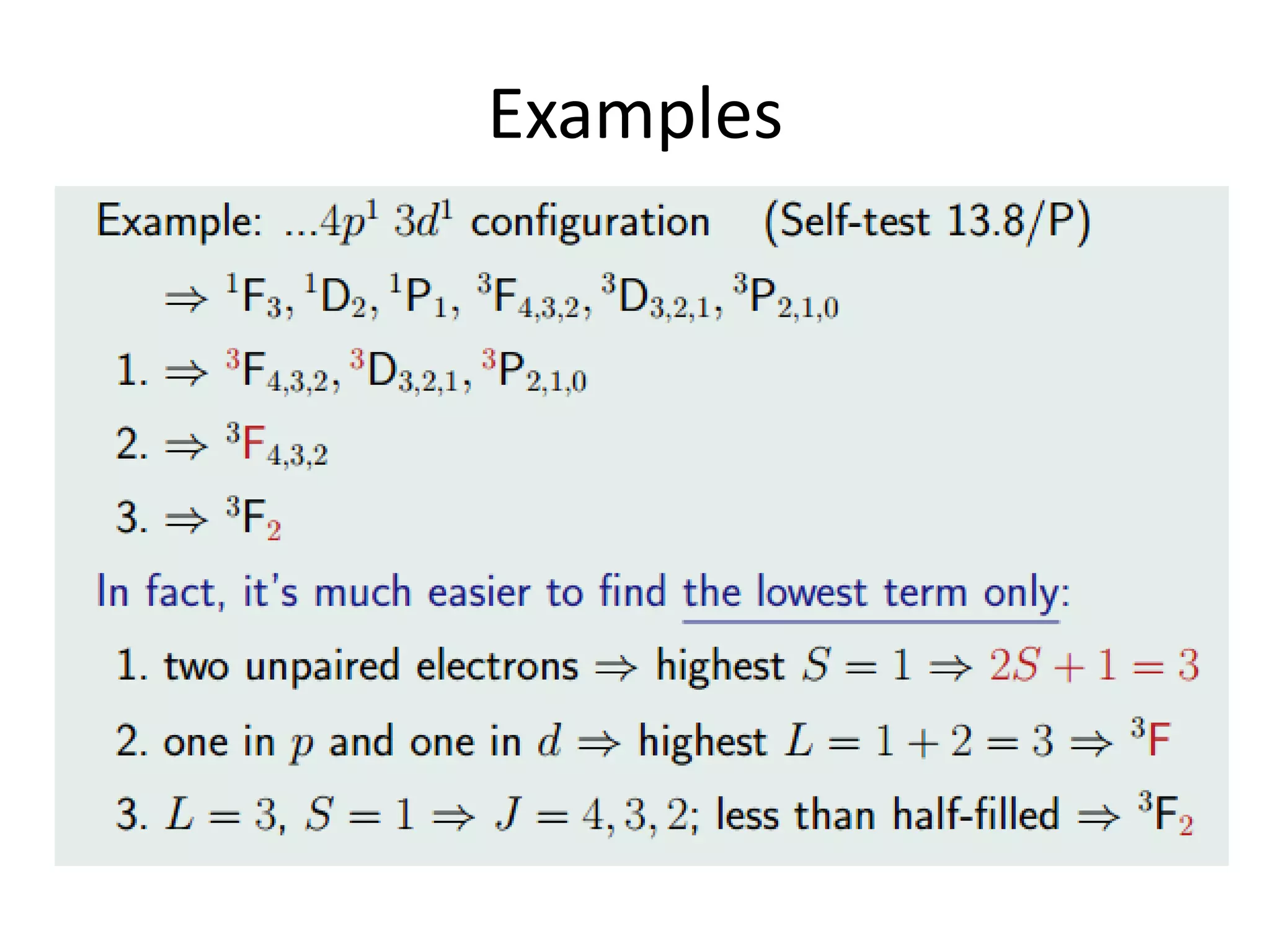
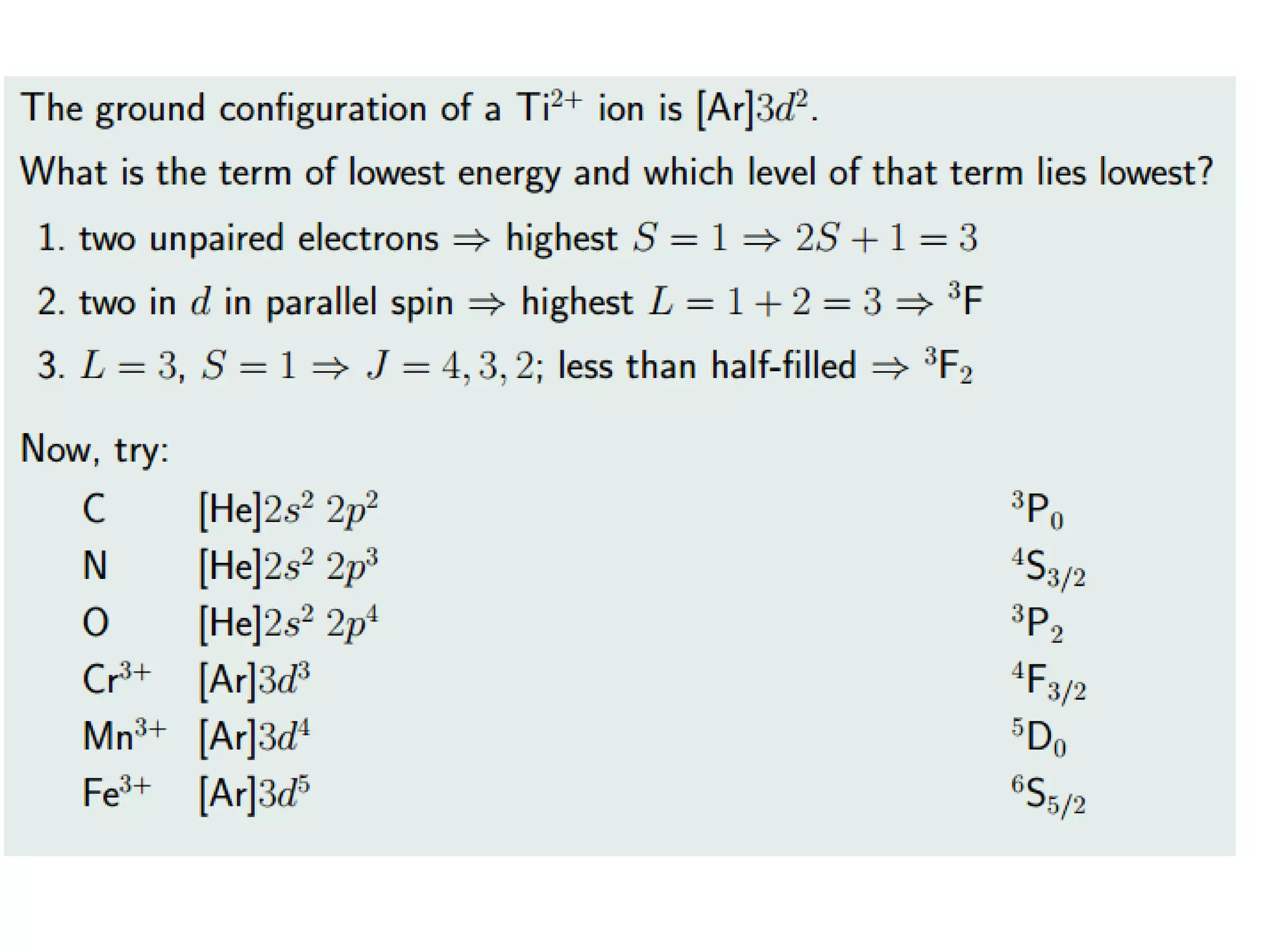
![“normal Spinel” vs. “inverse Spinel”
Ni(II) Mn(III)2O4 Mn(III) [Ni(II)Mn(III)] O4
2 Mn3+ in Oh:
= d4 HS
CFSE= 3*(-2/5o) + 3/5o
= -12.600 cm-1
For 2 Mn ->
-25.200 cm-1
(tetrahedral Ni(II):
E = 4*(-3/5 t) + 4*(2/5 t)
= -4/5 t = -4/5 * 0.44 * o
= -3000 cm-1 )
1 Mn3+ in Oh:
= d4 HS
CFSE= -12.600 cm-1
1 Ni2+ in Oh:
= d8 HS
CFSE= 6*(-2/5 o)+ 2*(3/5o)
= (-12/5 + 6/5)o (+ 3P)
= -6/5 o = -10.200 cm-1
-> total: 22.800 cm-1
(tetrahedral Mn(III):
E = 2*(-3/5t) + 2*(2/5t)
= = -2/5 t = -2/5 * 0.44 * o
= -3700 cm-1 )
=> We can look only on the
octahedral configurations
to decide !](https://image.slidesharecdn.com/propertiesofcoordinationcompoundspart2-180303081212/75/Properties-of-coordination-compounds-part-2-3-2048.jpg)

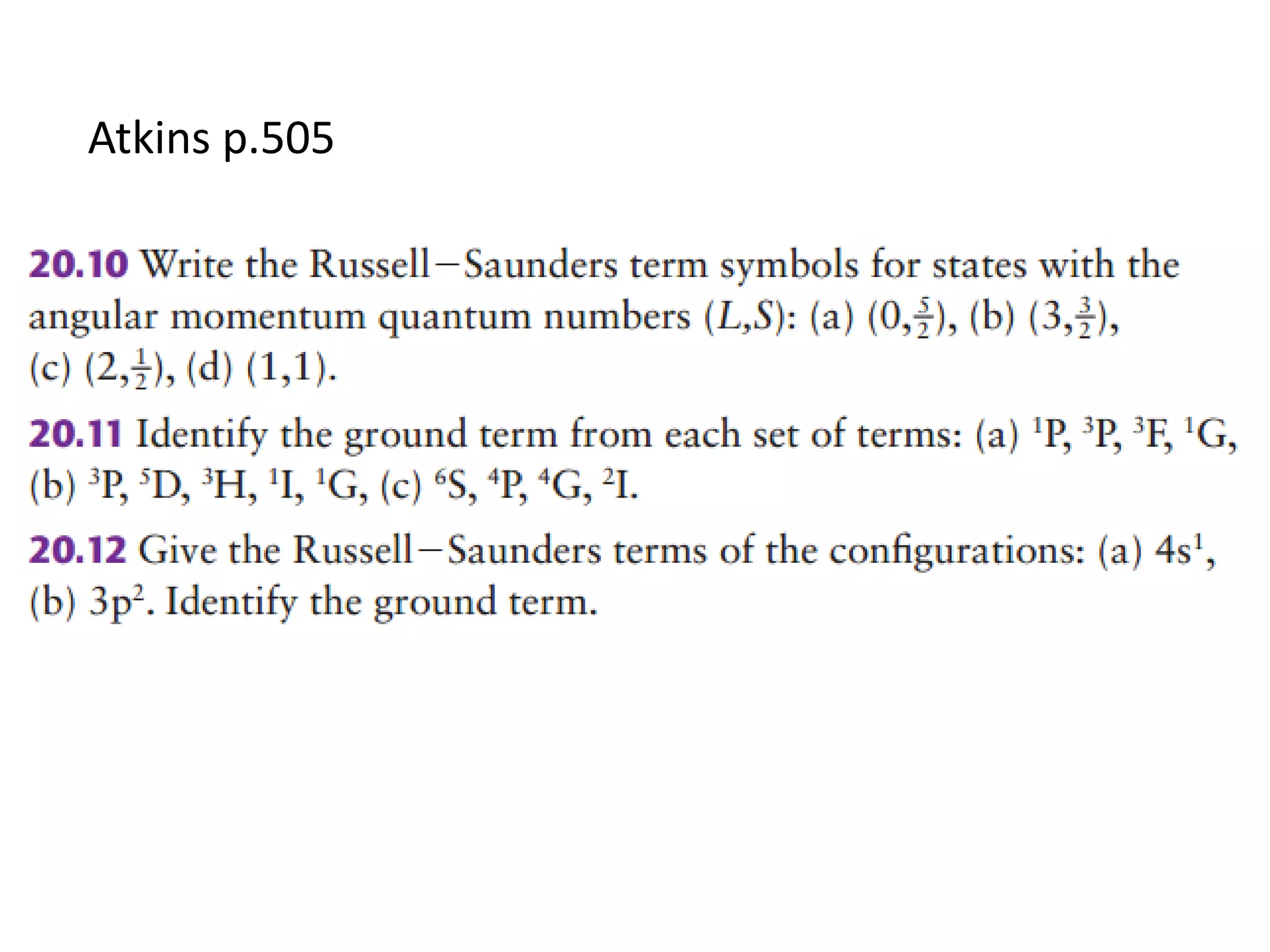
![Find the electron configuration from the following
observations:
(a) μeff for [Cr(NH3)6]Cl2 is 4.85 μB.
(b) μeff for [V(NH3)6]Cl2 is 3.9 μB.
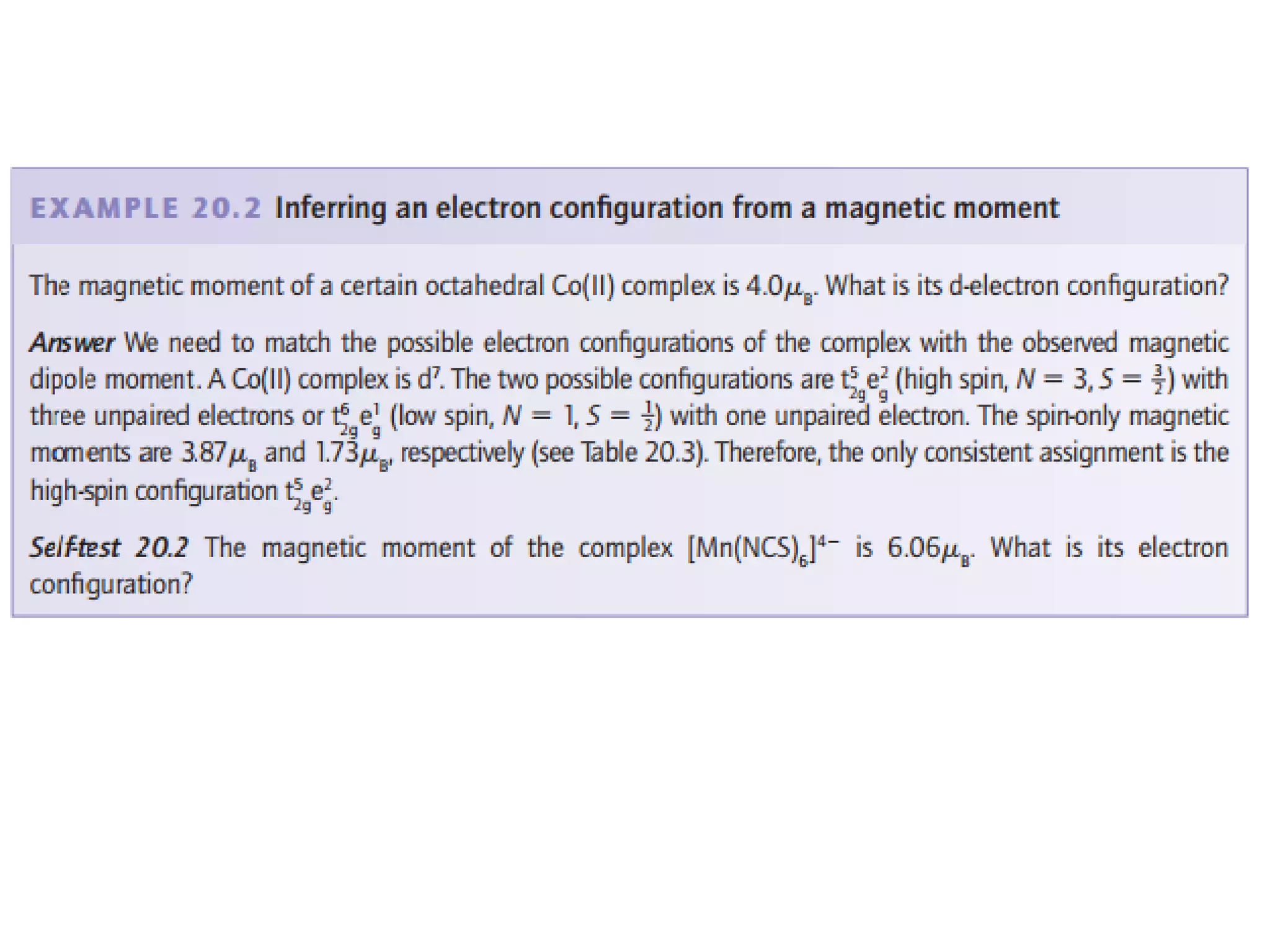
(c) μeff for a Co(II) complex is 4.0 μB.
(d) μeff for [Mn(NCS)6]4(-) is 6.06 μB.](https://image.slidesharecdn.com/propertiesofcoordinationcompoundspart2-180303081212/75/Properties-of-coordination-compounds-part-2-20-2048.jpg)

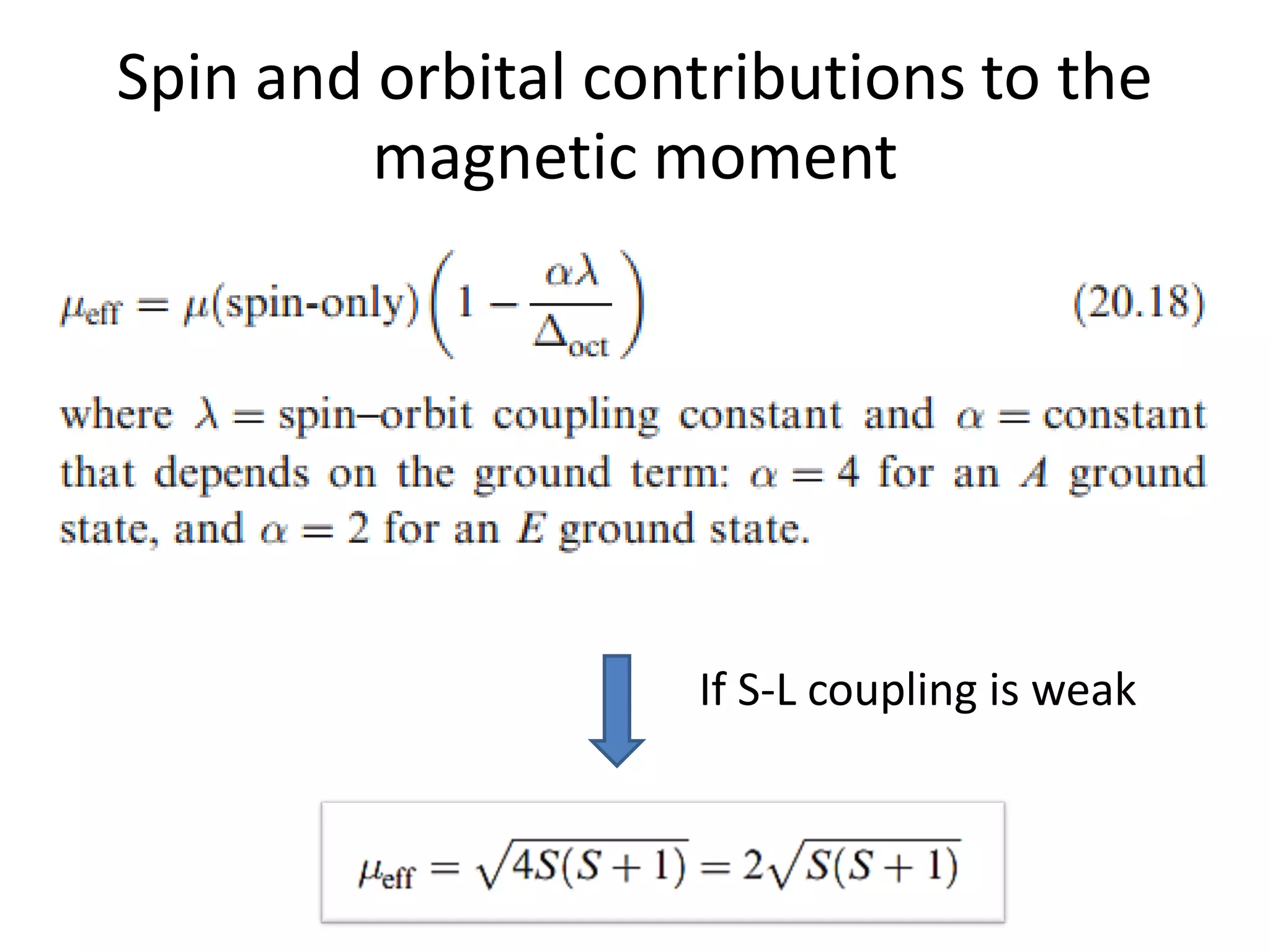
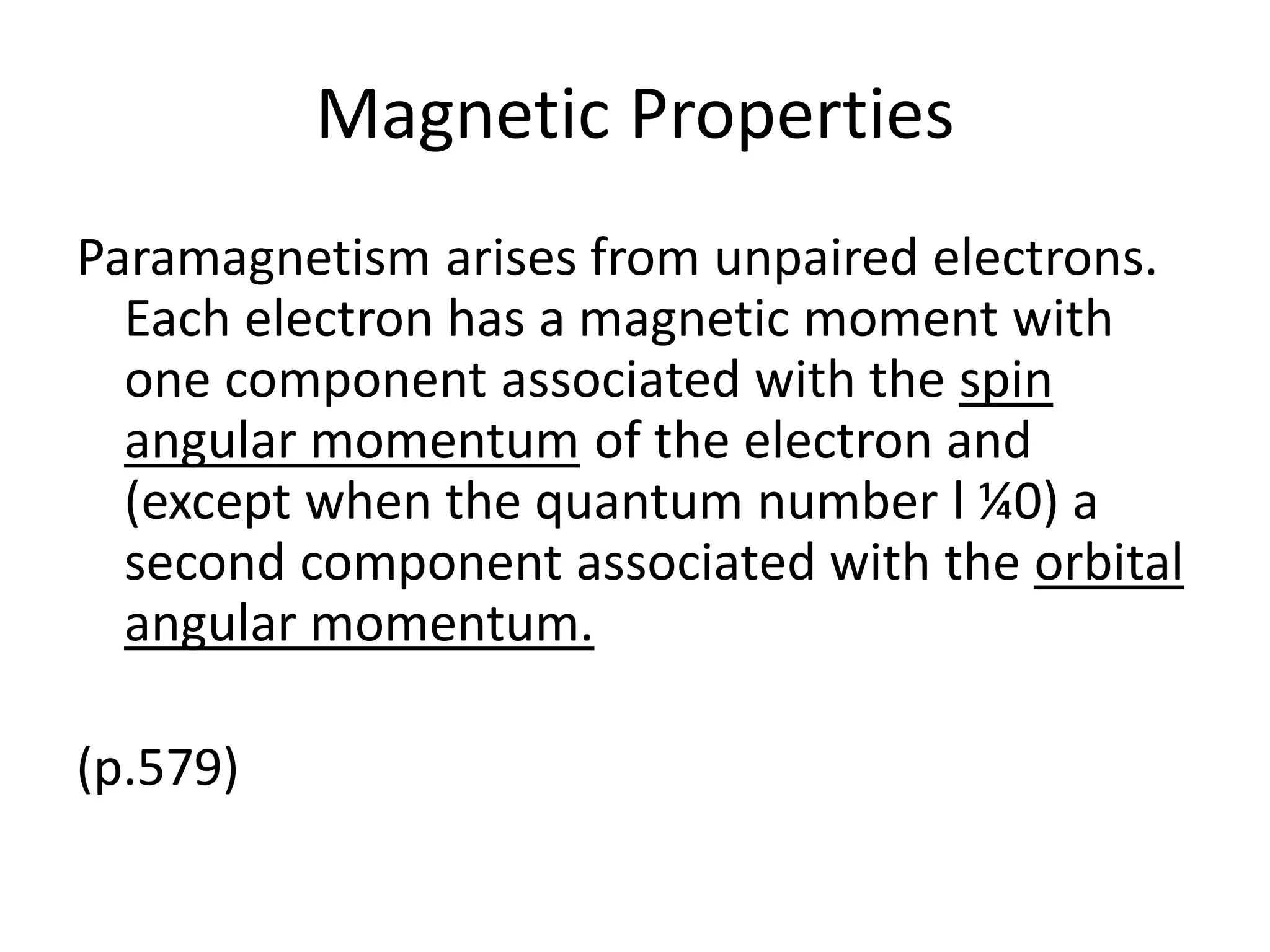
![Deviations from spin-only formula
Example:
[Fe(CN)6]3- has μ = 2.3μB
which is between low- and high-spin calculated
(check this out)](https://image.slidesharecdn.com/propertiesofcoordinationcompoundspart2-180303081212/75/Properties-of-coordination-compounds-part-2-27-2048.jpg)