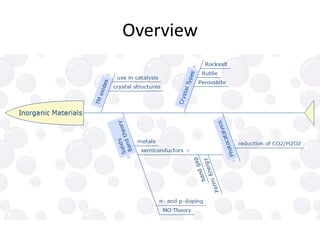
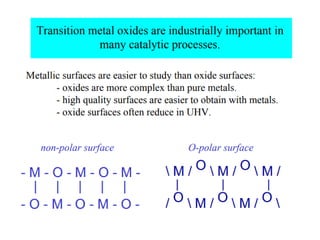
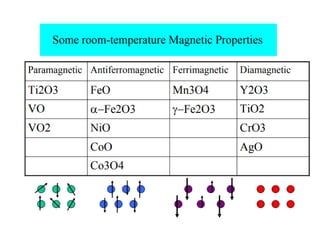
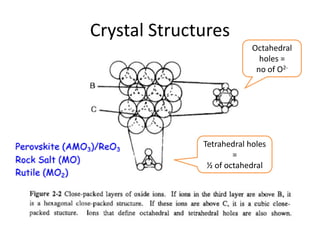
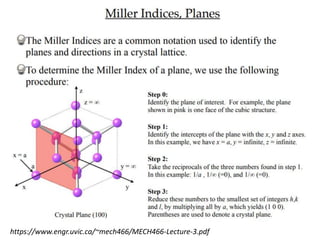
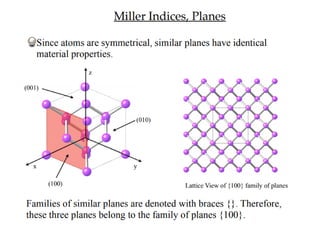
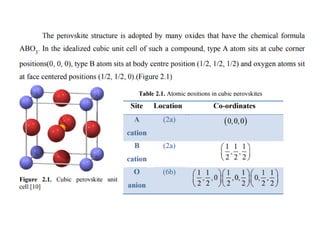
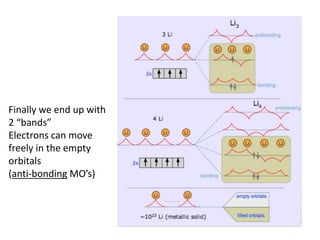
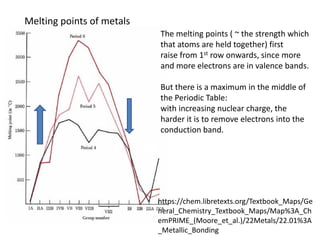
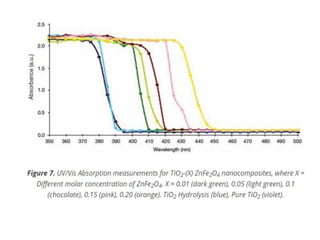
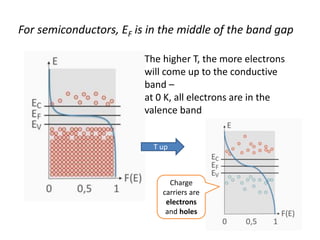
1) The document discusses the properties of inorganic materials such as transition metal oxides and their crystal structures including octahedral and tetrahedral holes. It also covers band theory and how it relates to the electrical, optical, and magnetic properties of solids.
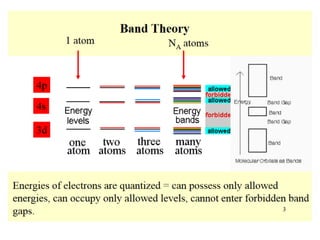
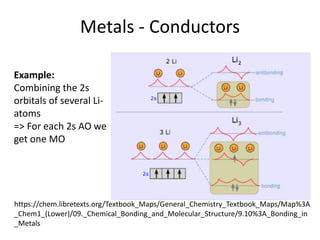
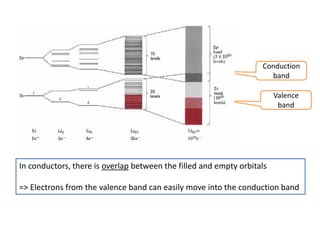
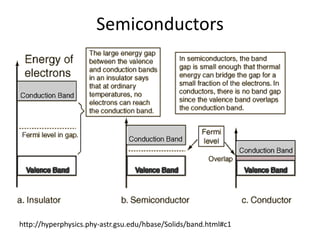
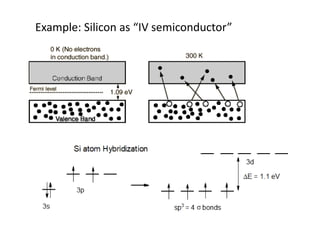
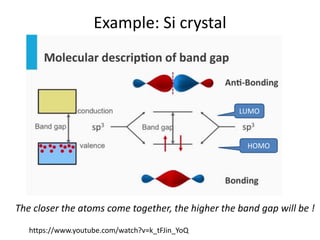
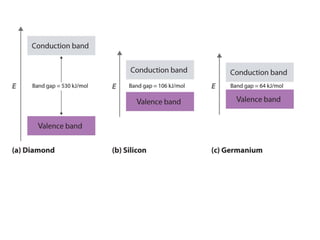
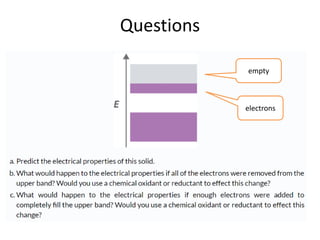
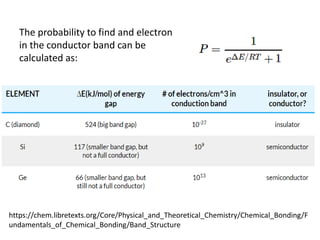
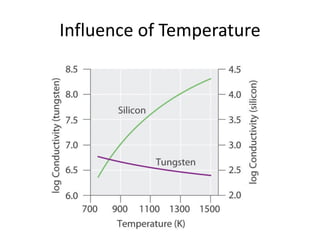
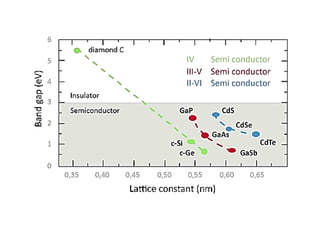
2) Band theory is used to explain the differences between conductors, semiconductors, and insulators. In conductors there is overlap between the filled valence band and empty conduction band allowing electrons to move freely. Semiconductors like silicon have a small band gap that can be overcome by thermal excitation.
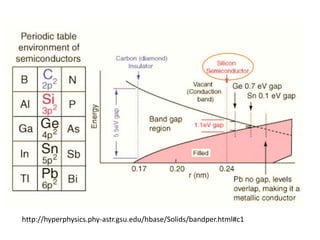
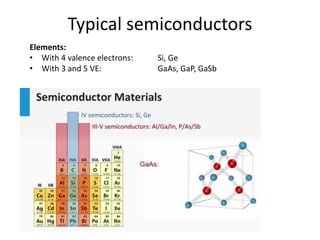
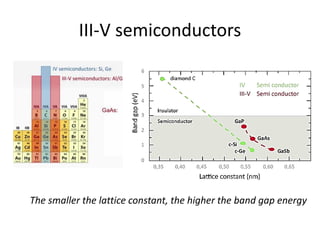
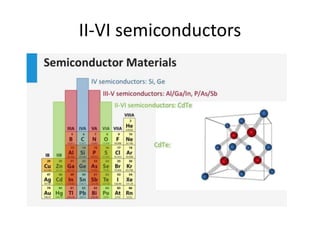
3) Common semiconductor materials are elements in columns IV like silicon and germanium, and III-V compounds like gallium arsenide.