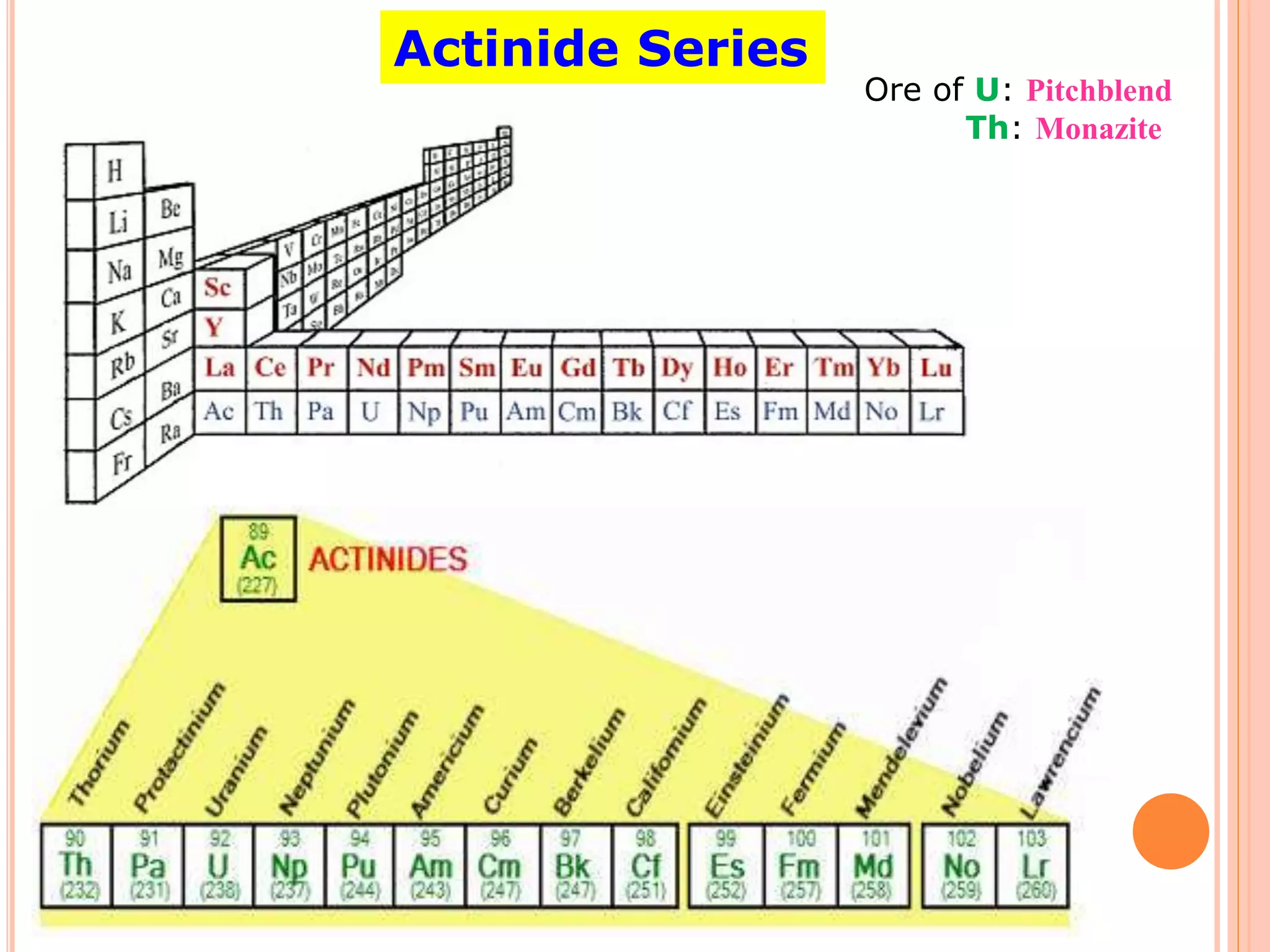
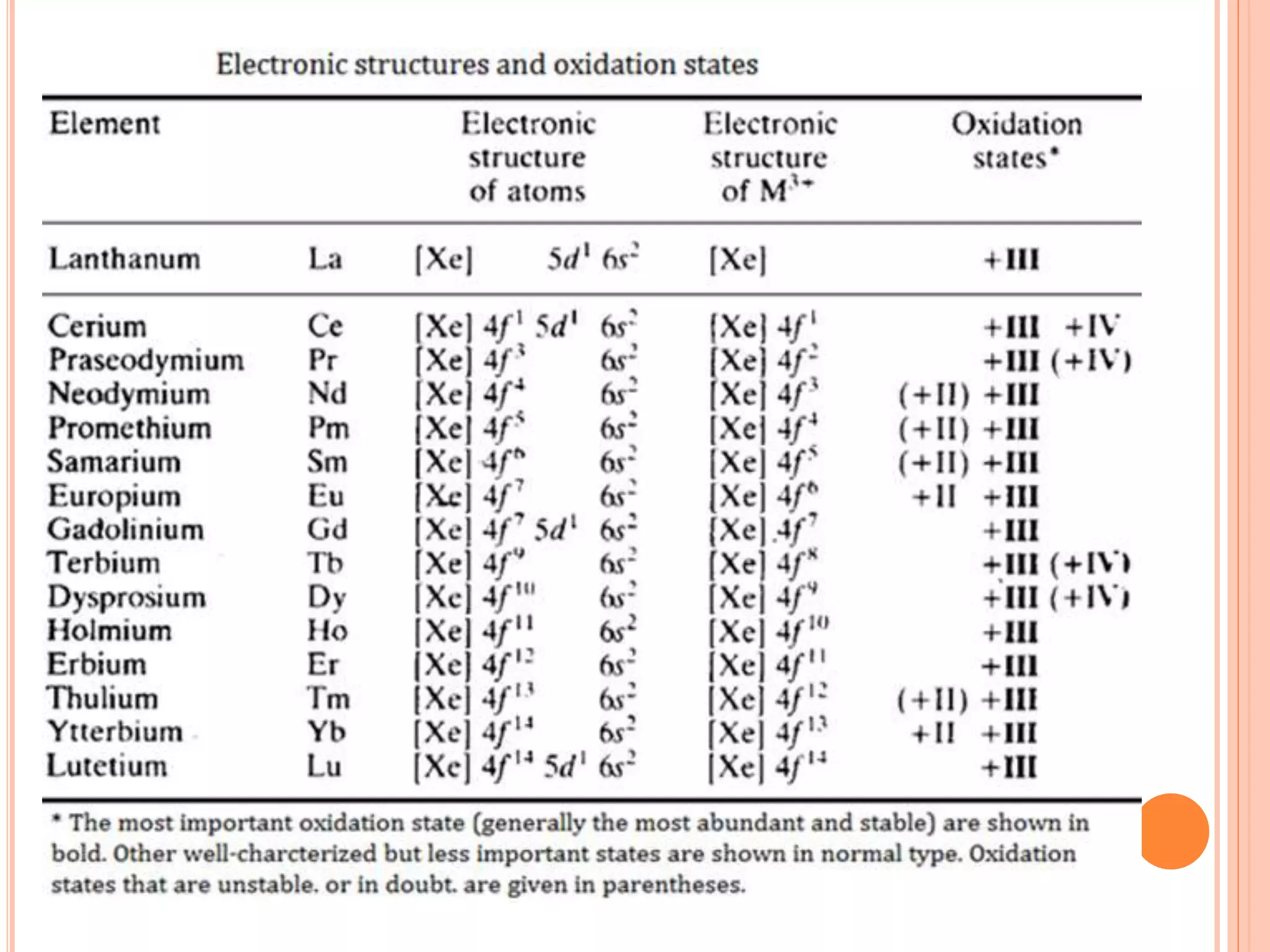
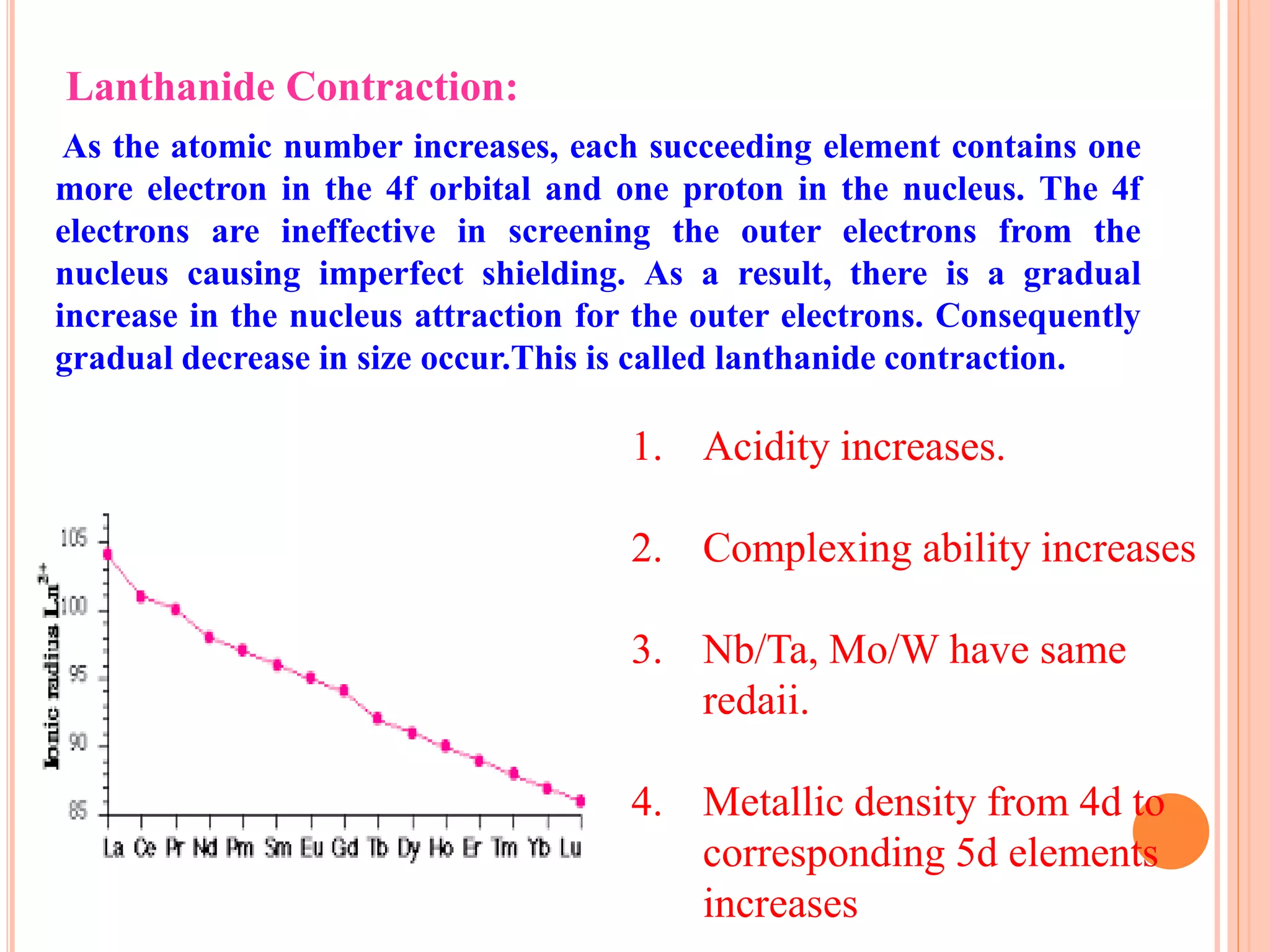
The document discusses lanthanide and actinide chemistry, highlighting the historical discovery of lanthanides and their unique properties, such as oxidation states, magnetic susceptibilities, and fluorescence. It elaborates on the challenges of separating lanthanides due to their similar characteristics and compares them with actinides, noting similarities in f-orbital filling and differences in oxidation states and radioactivity. The document also covers various chemical behaviors, bonding nature, and extraction techniques related to these elements.













![MAGNETIC PROPERTIES
Lanthanides have very high magnetic susceptibilities due
to their large numbers of unpaired f-electrons.
The lanthanoid ions other then the f 0 type (La3+ and Ce3+) and
the f14 type (Yb2+ and Lu3+) are all paramagnetic. The
paramagnetism rises to the maximum in neodymium.
The strongest known magnets contain lanthanides
(eg. Nd-Fe-B, Sm-Fe-N, and Sm-Co).
Lanthanide complexes are used in MRI
(magnetic resonance imaging), eg. [Gd(III)(dtpa)]2-](https://image.slidesharecdn.com/lanthanideandactinidechemistry-201011155740/75/Lanthanide-and-actinide-chemistry-16-2048.jpg)