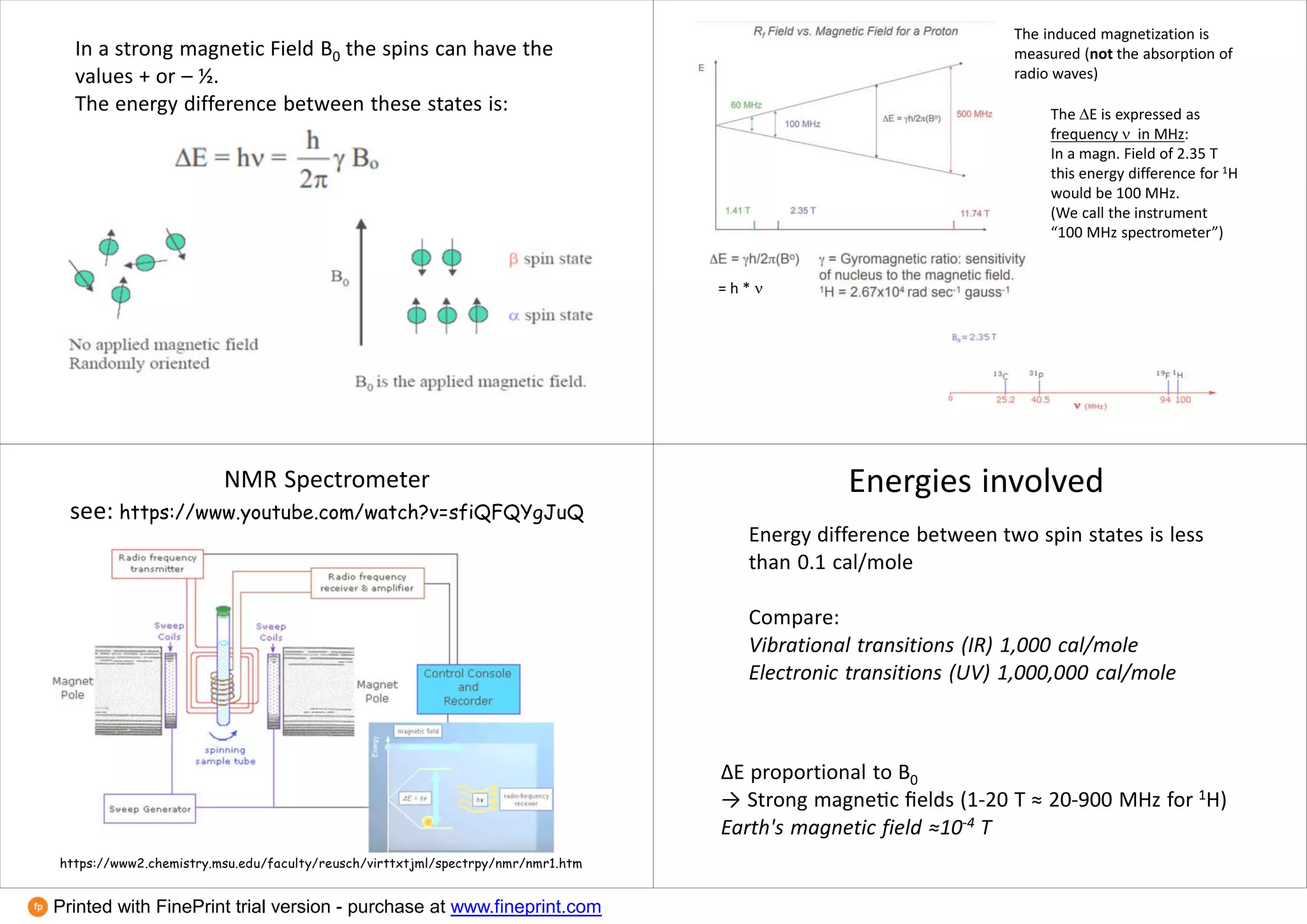
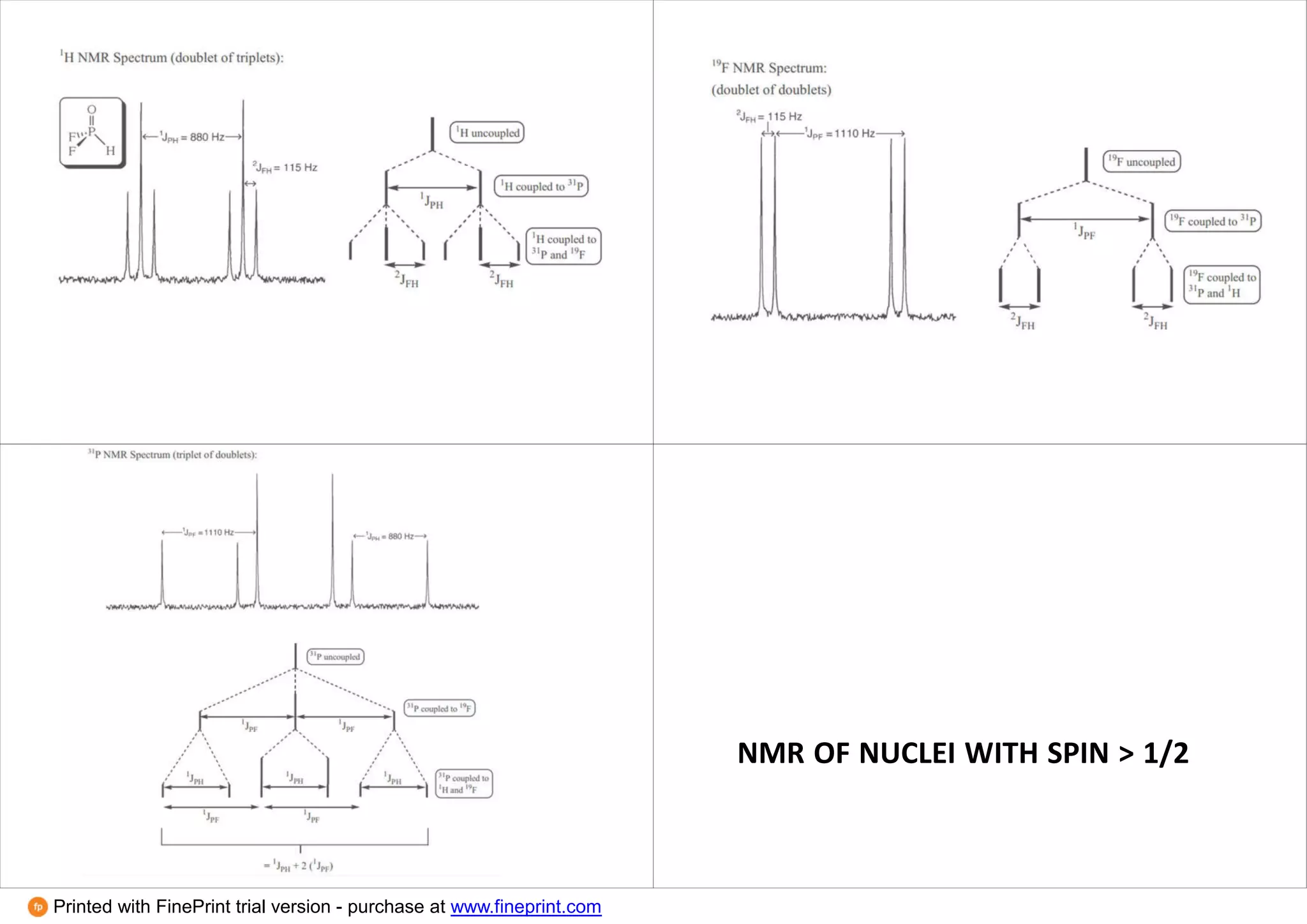
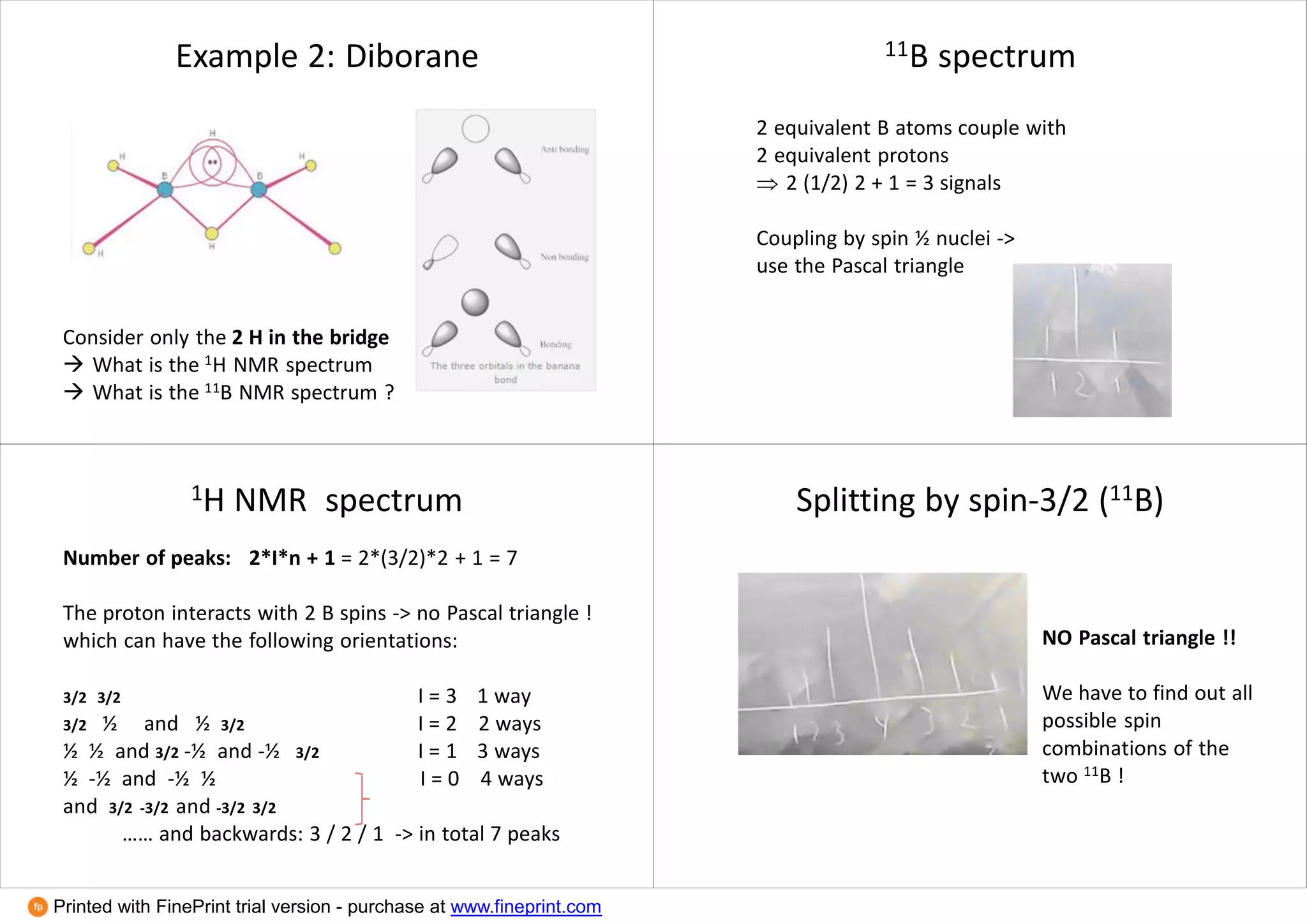
This document provides an overview of NMR spectroscopy techniques in inorganic chemistry. It discusses how the magnetic moments of electrons and nuclei can be quantified and how this allows NMR active nuclei to be detected. It explains the concepts of spin quantum numbers, resonance frequencies, chemical shifts, spin-spin coupling, and how these principles underpin 1H, 13C, 19F, 31P and other NMR experiments. Examples are given to illustrate NMR spectra for nuclei with different spin values and how dynamic NMR can provide information about molecular exchange rates.