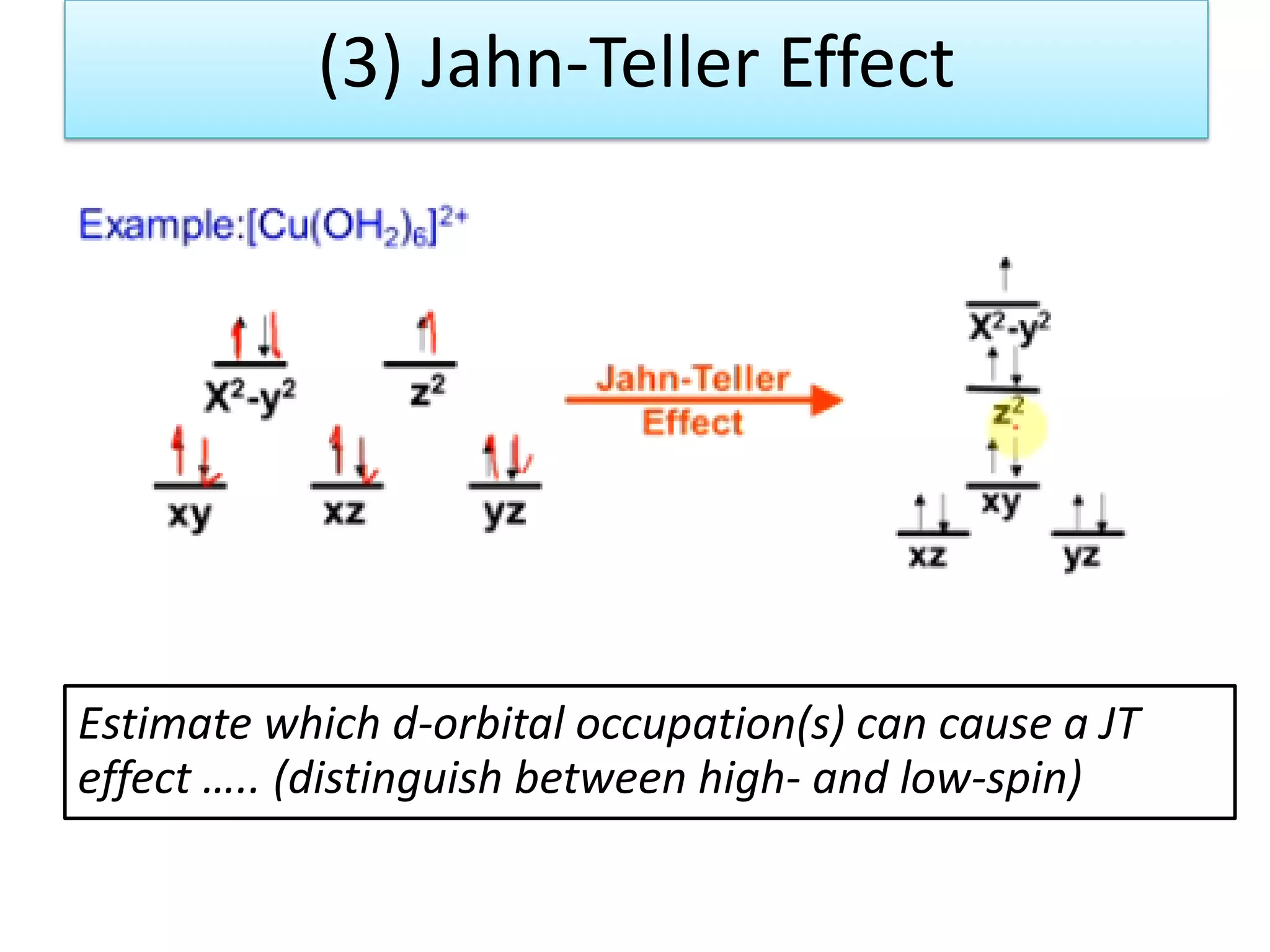
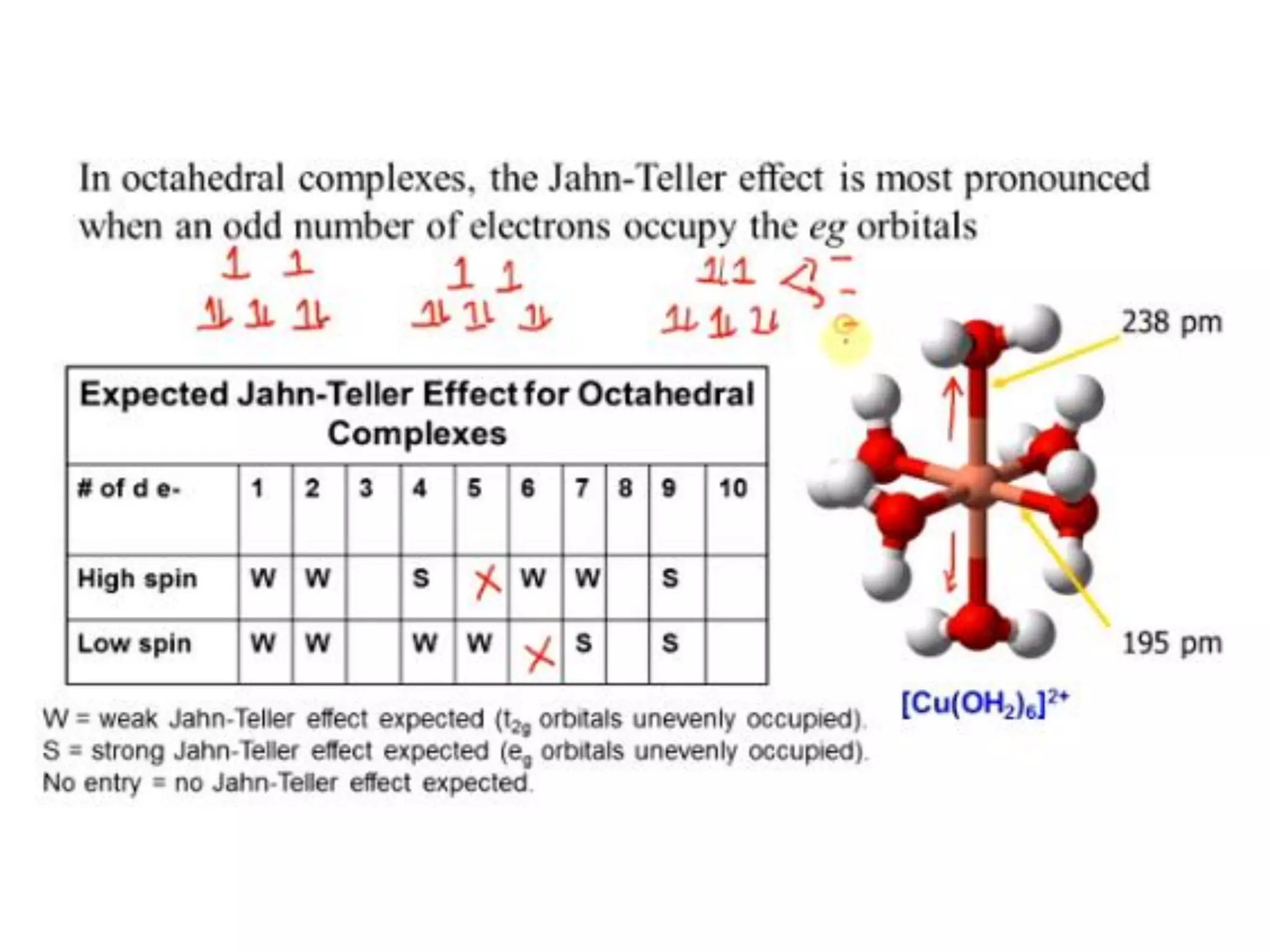
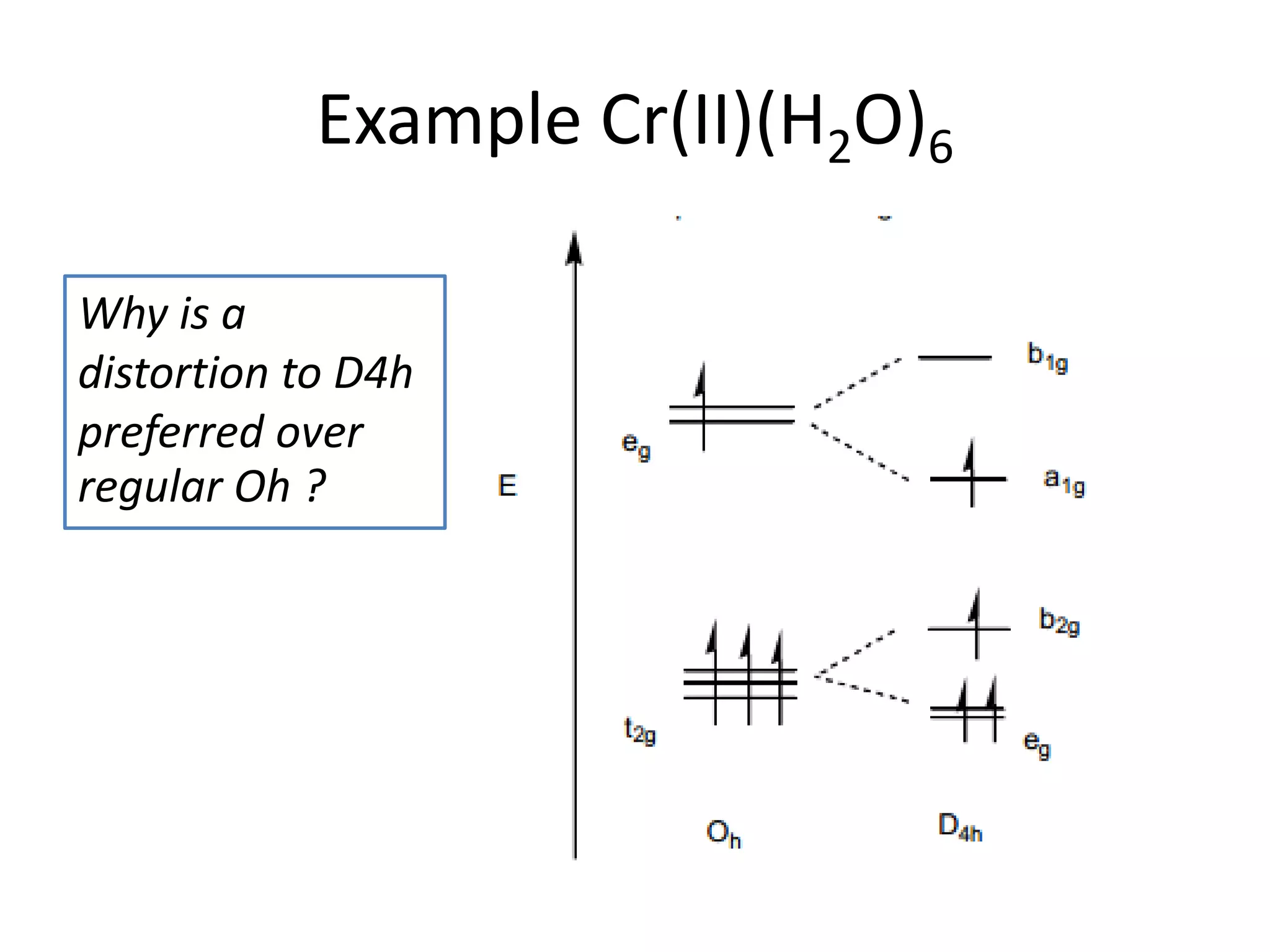
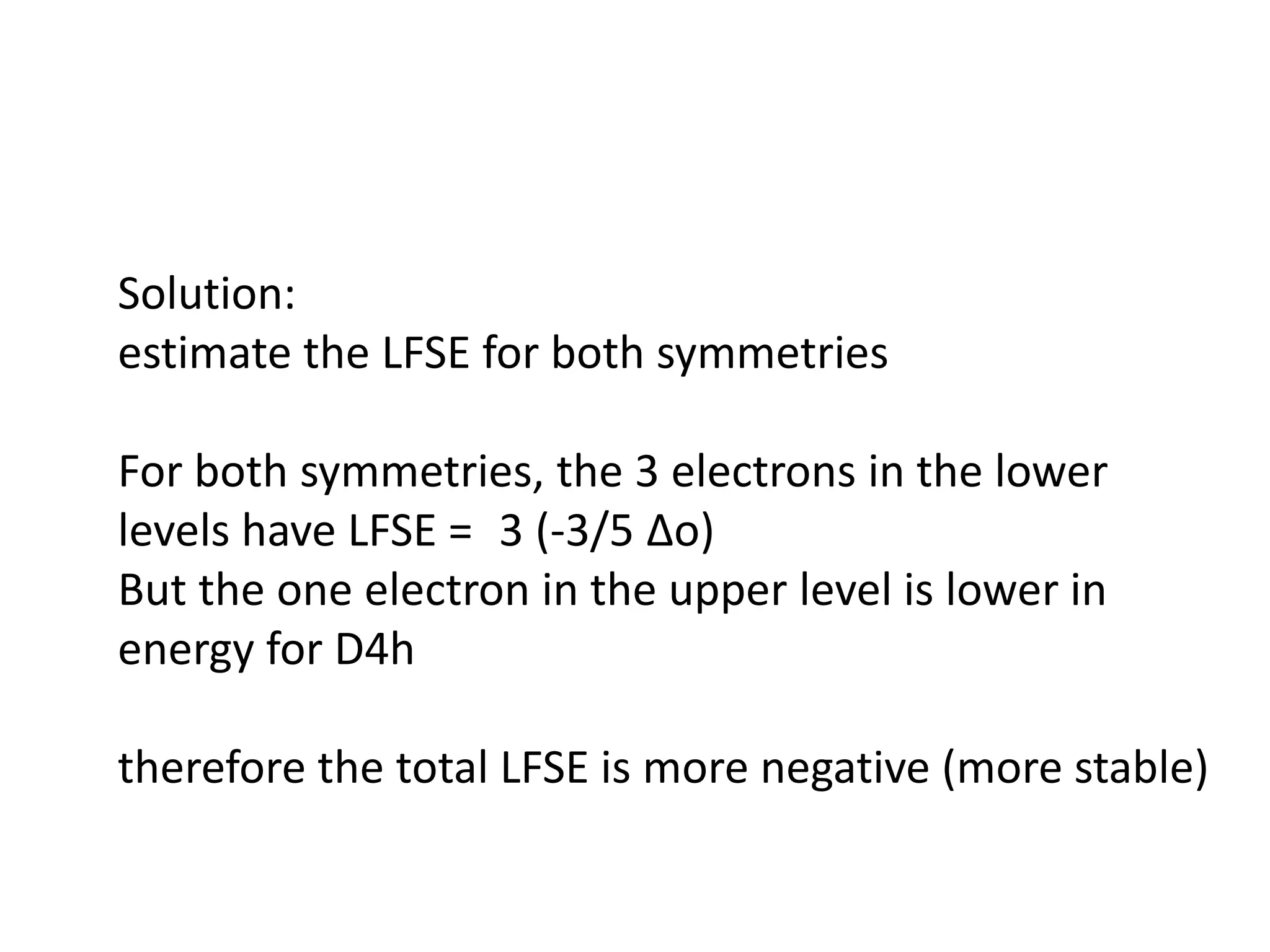
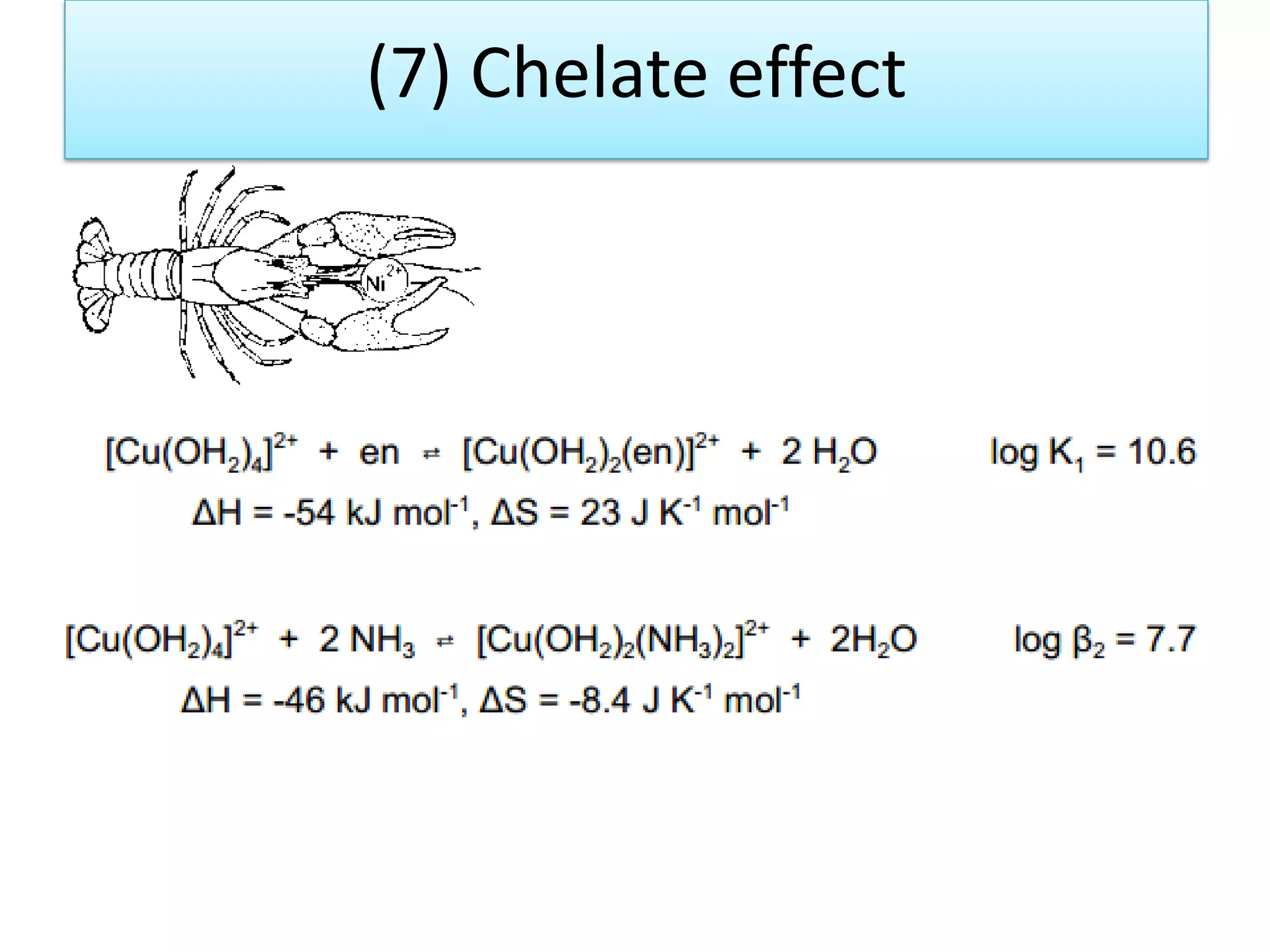
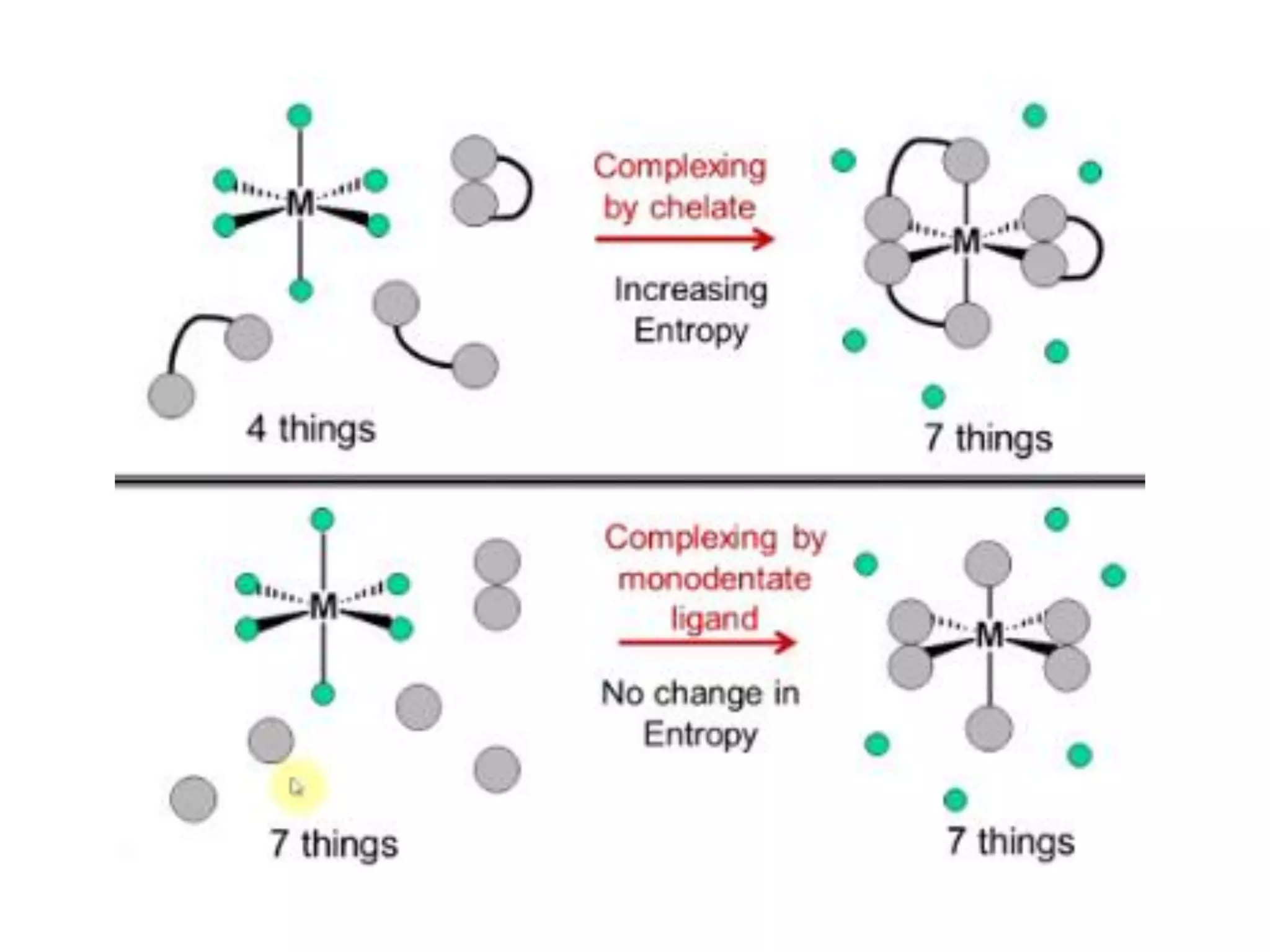
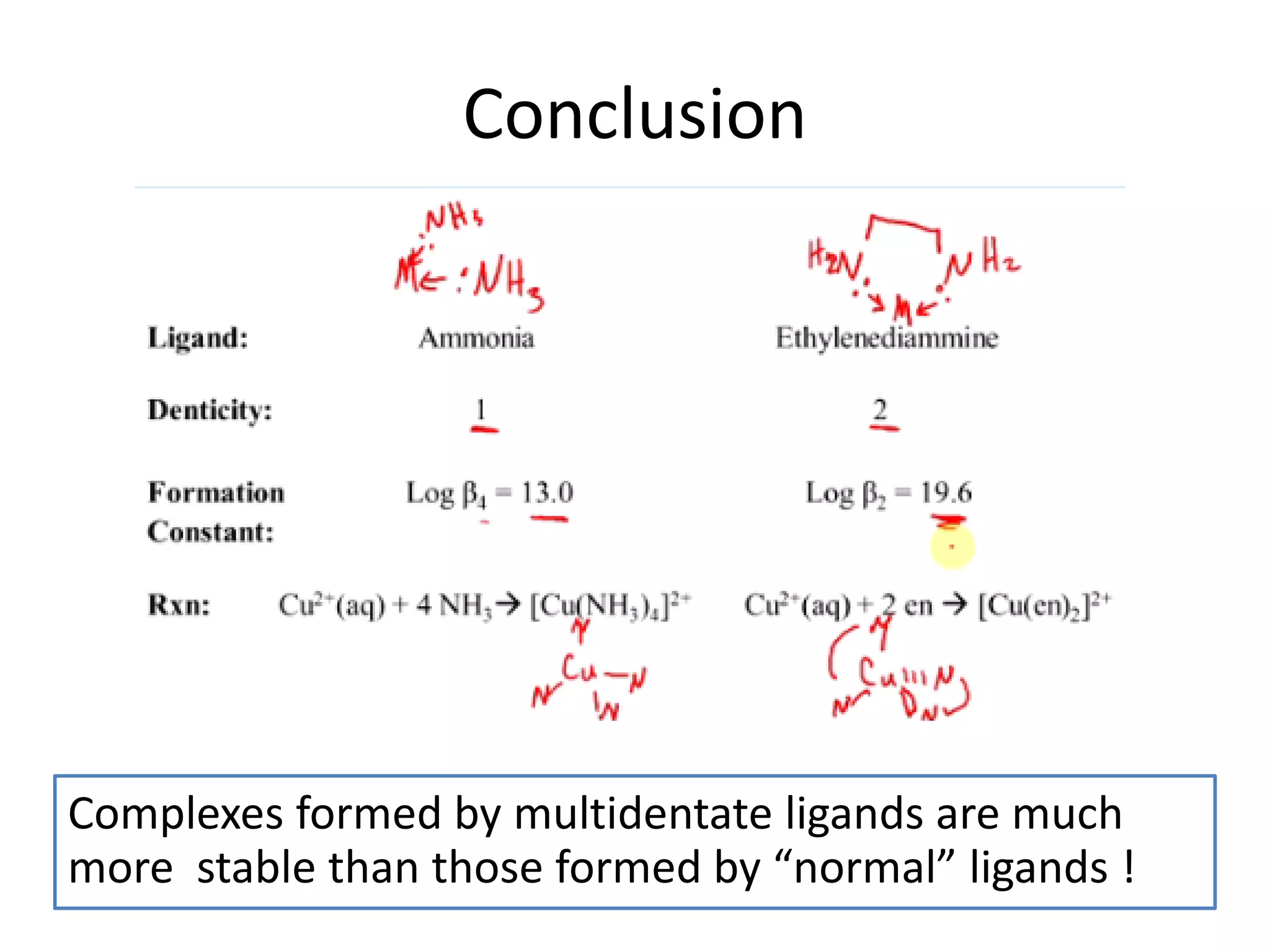
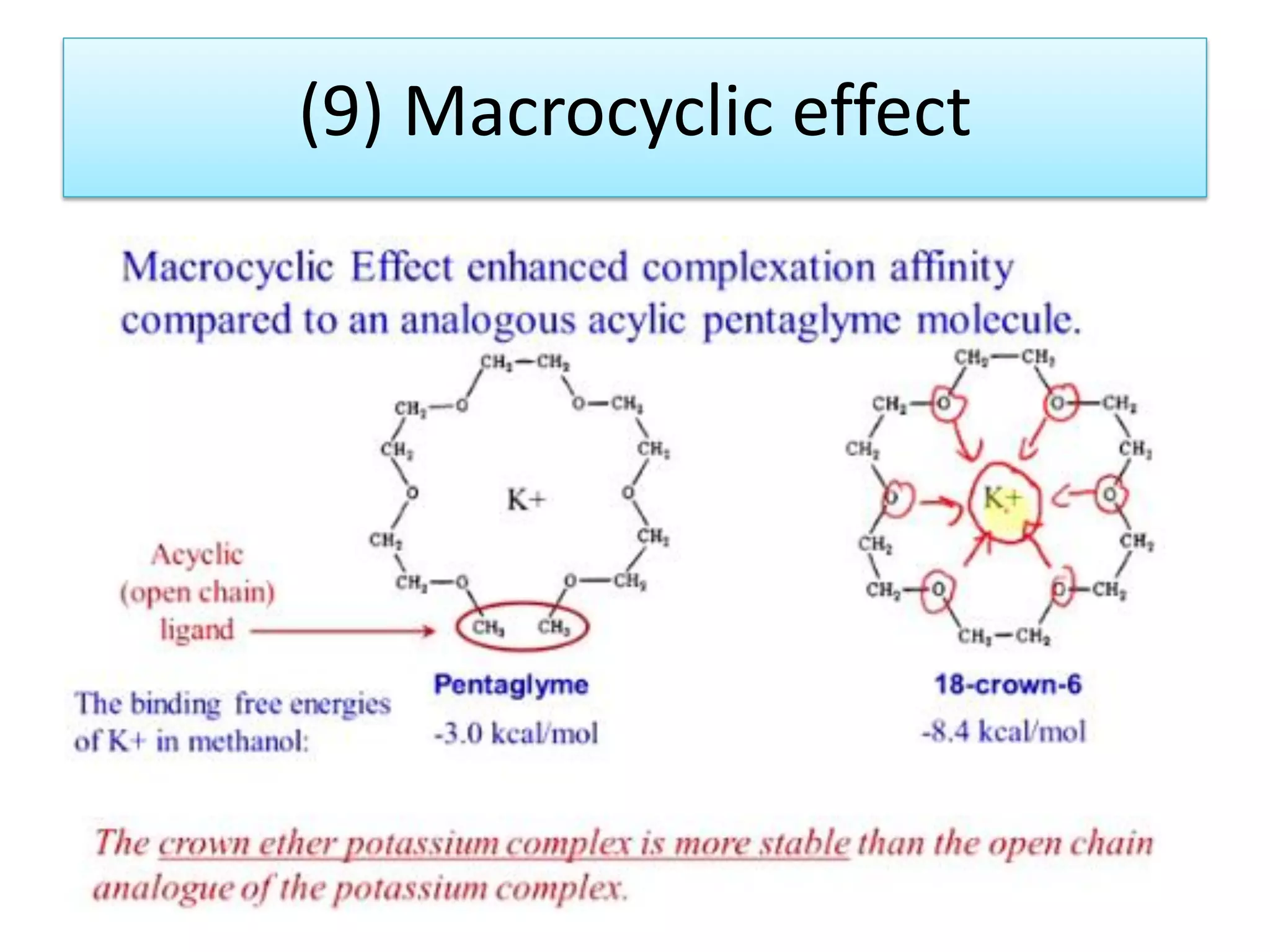
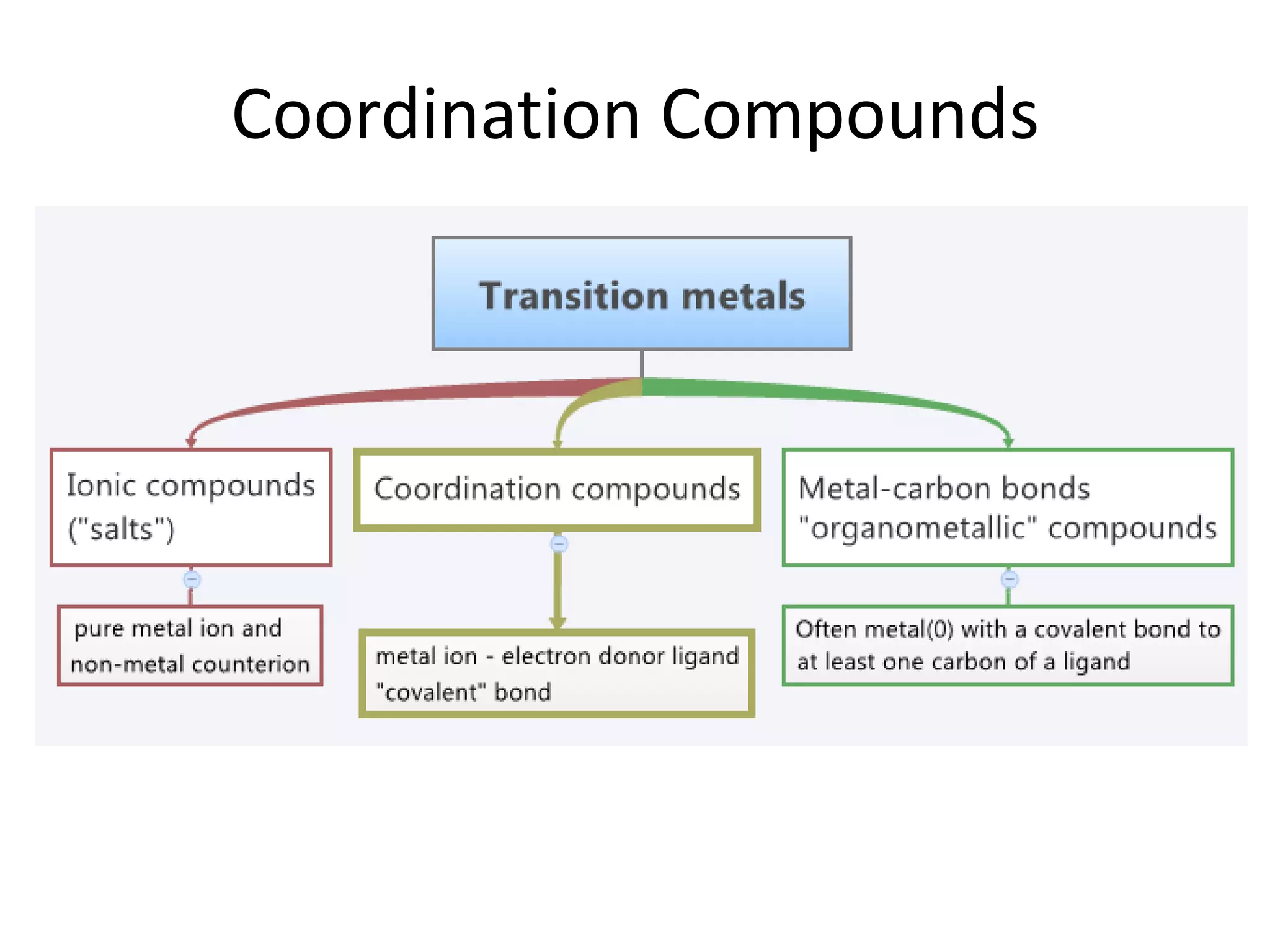
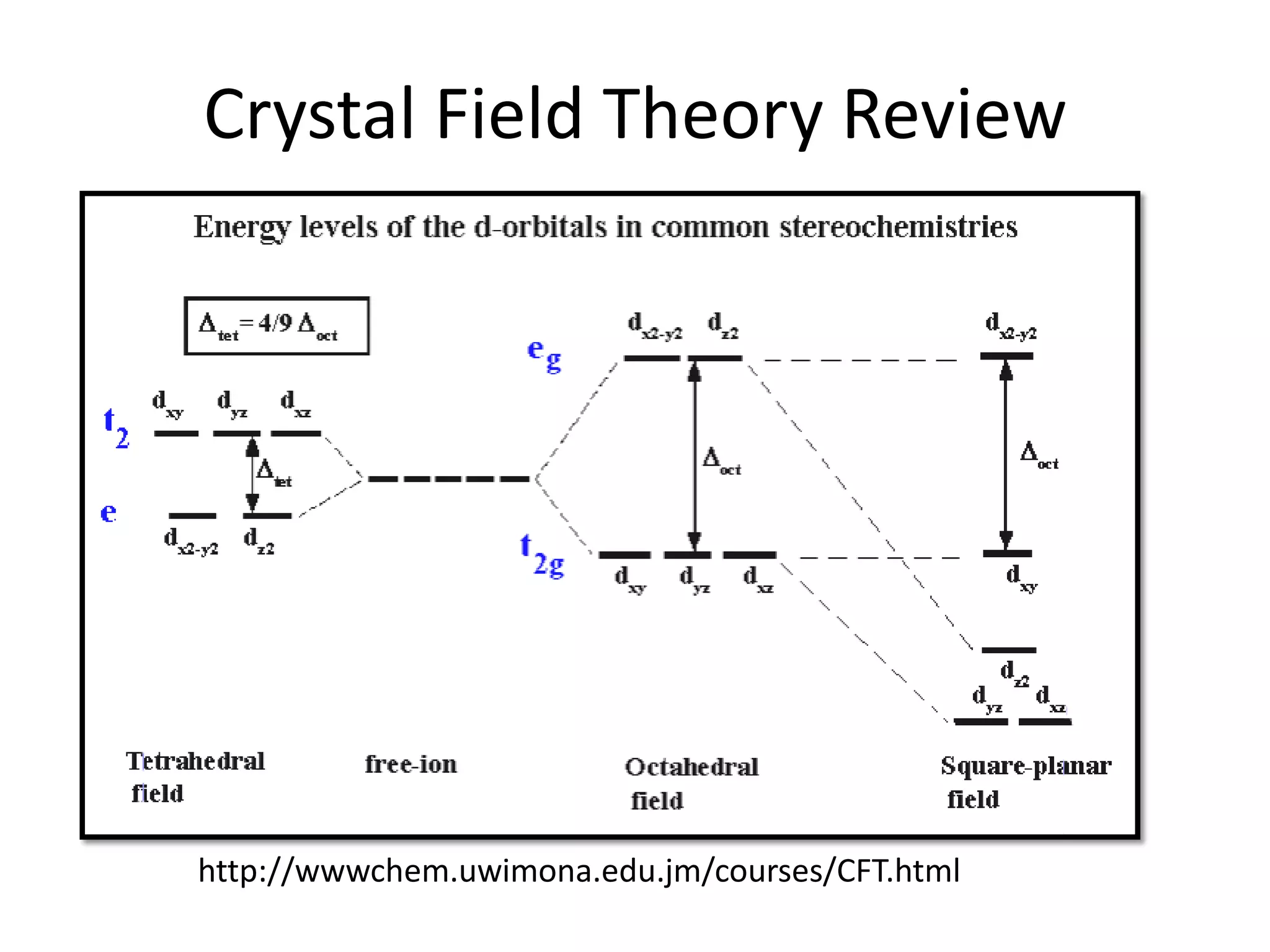
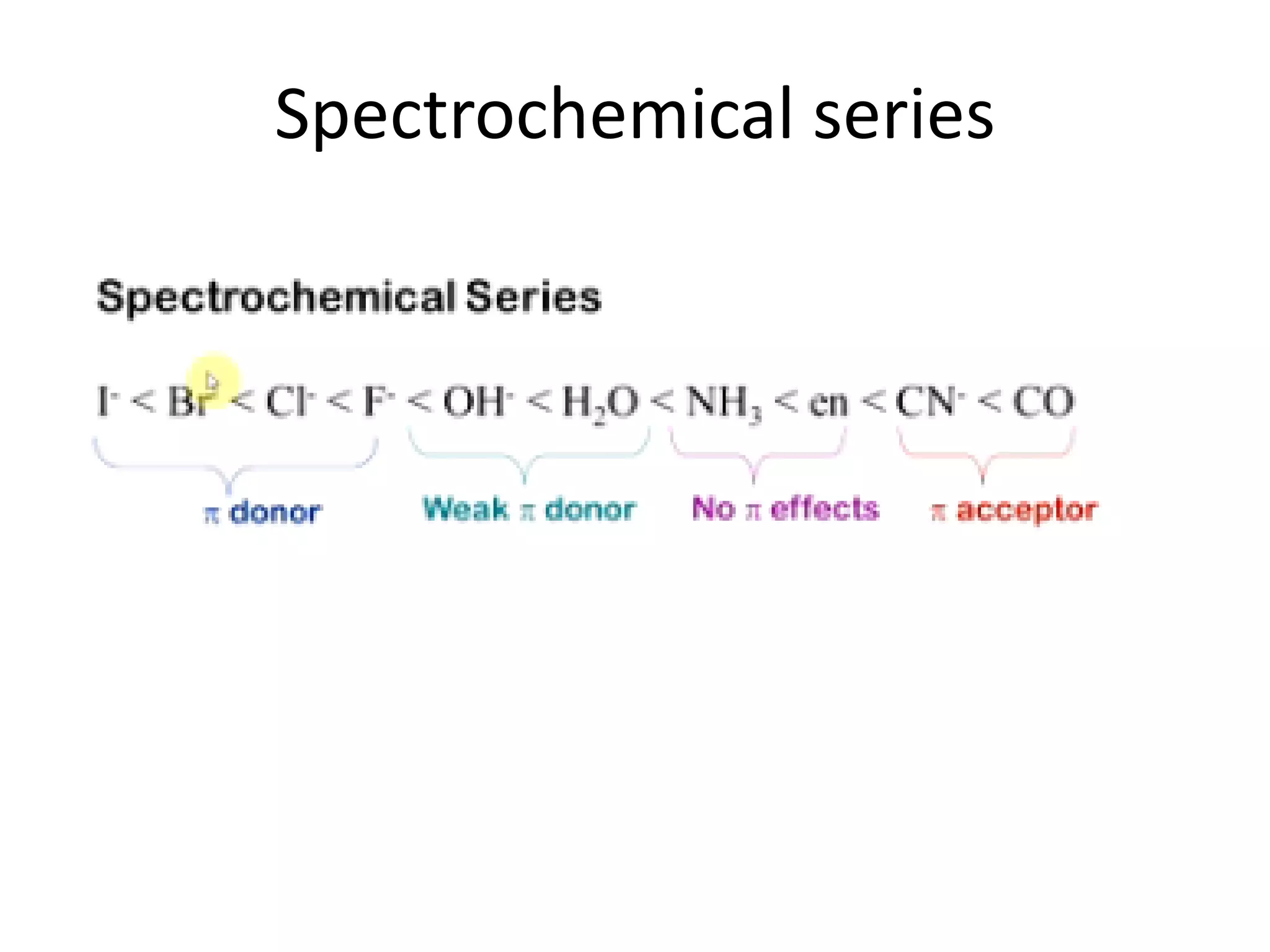
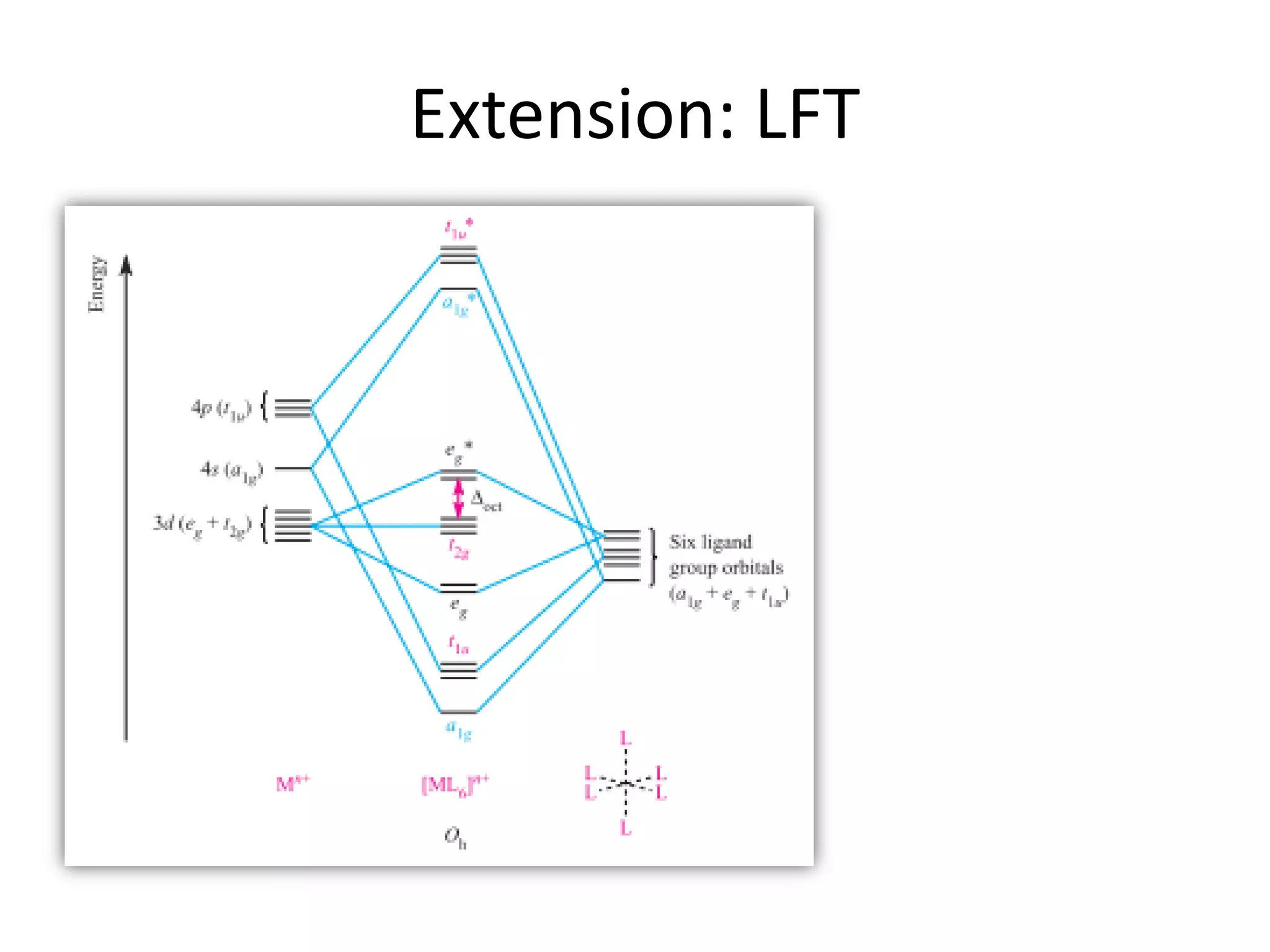
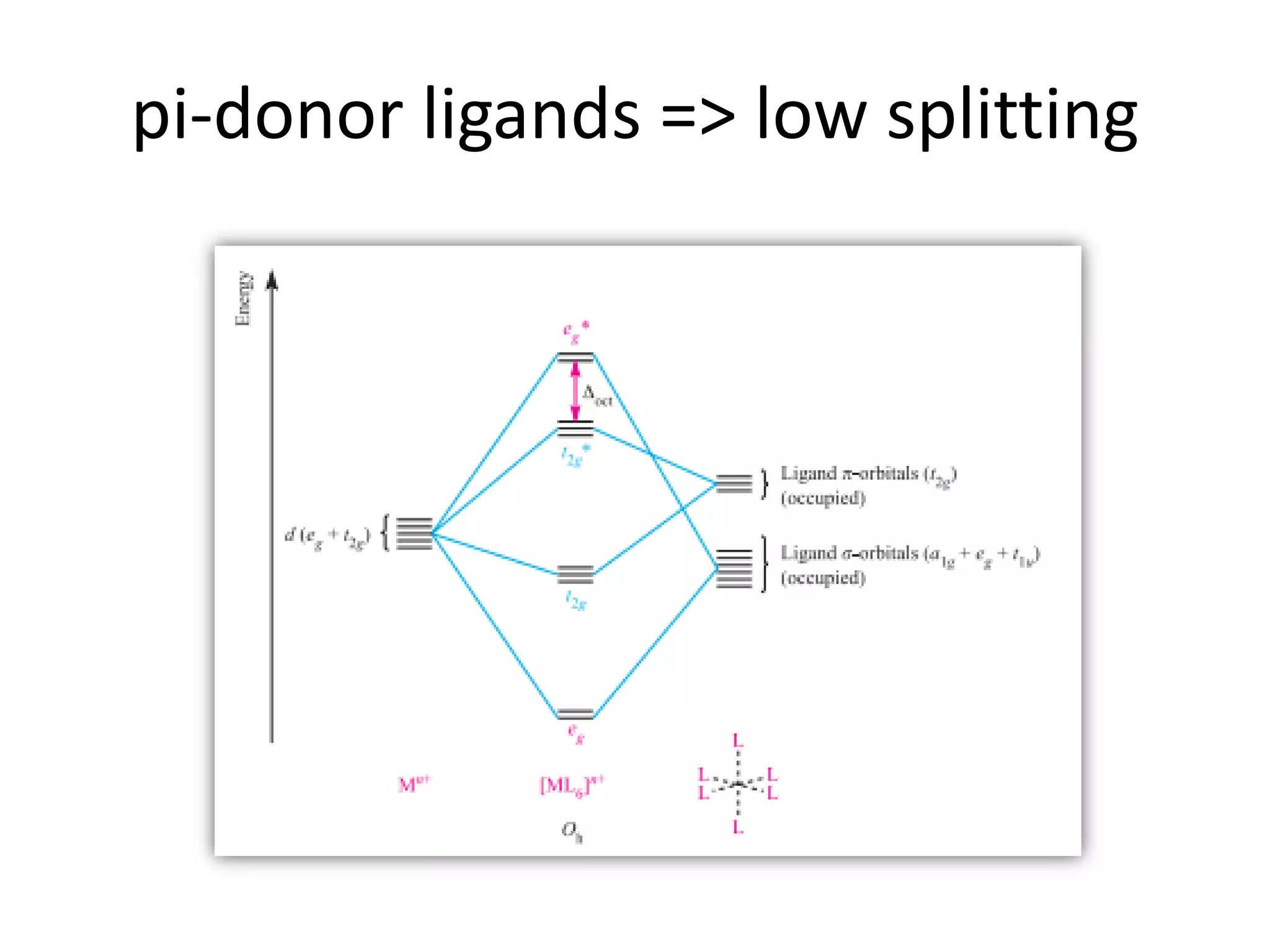
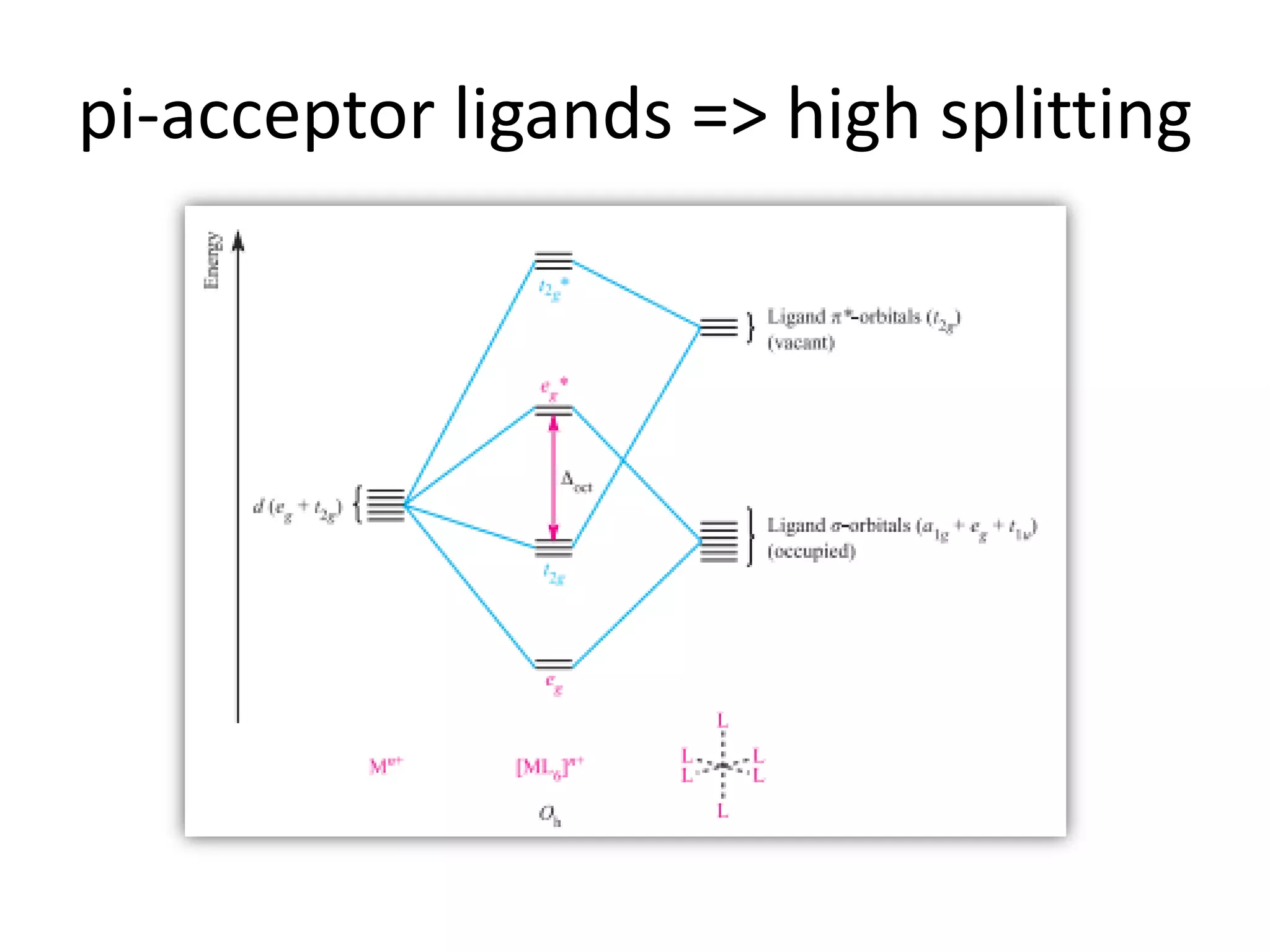
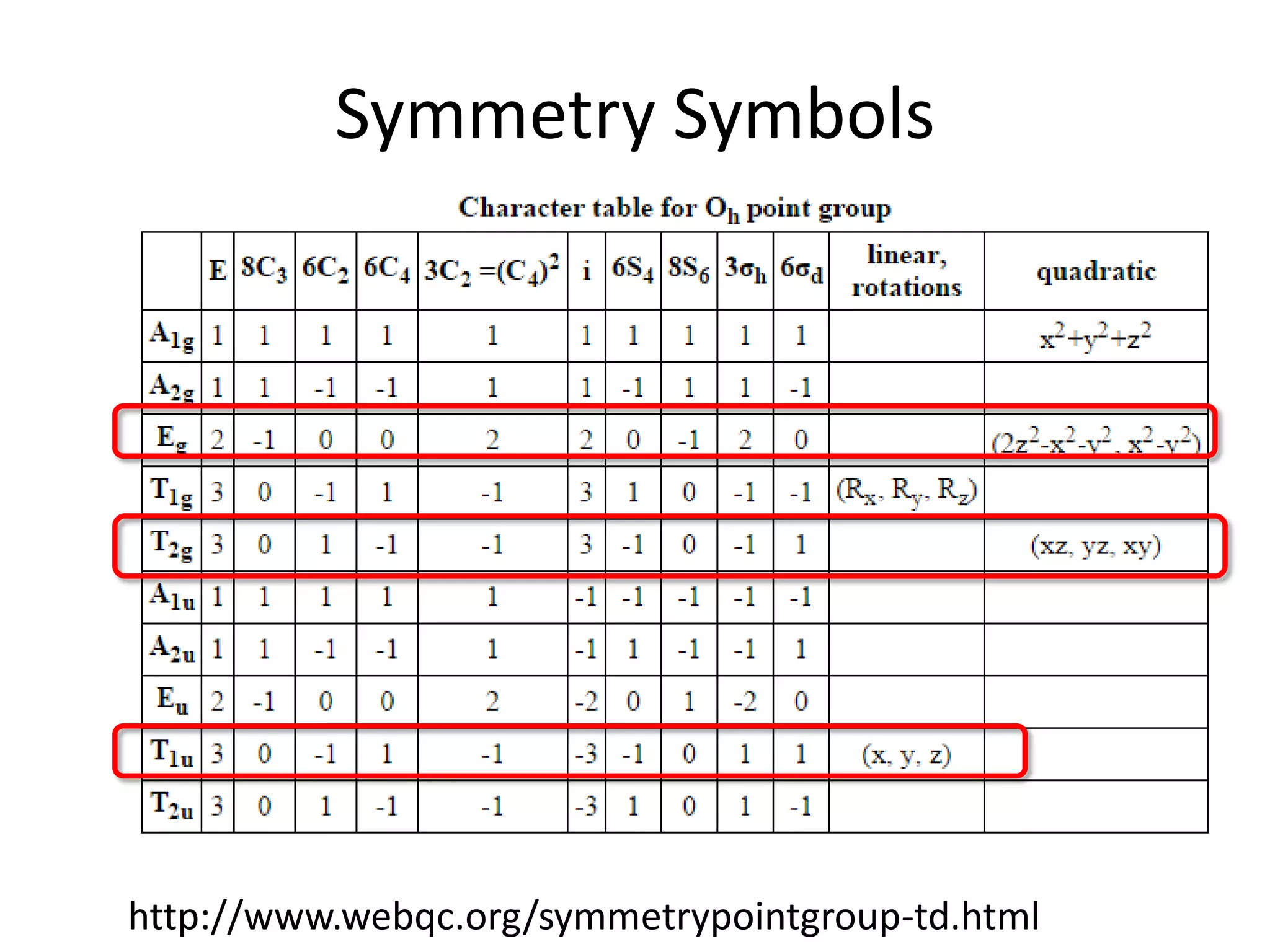
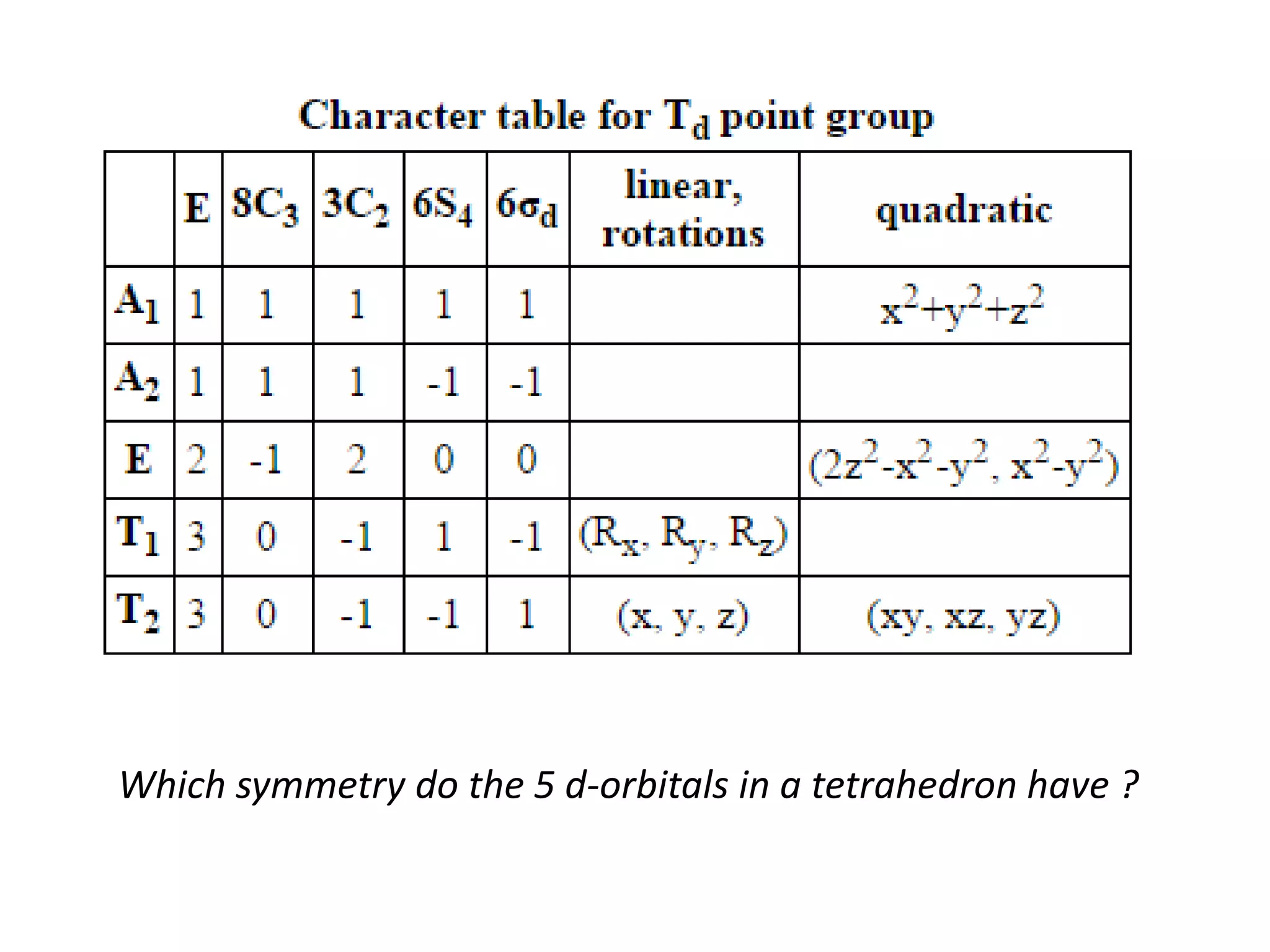
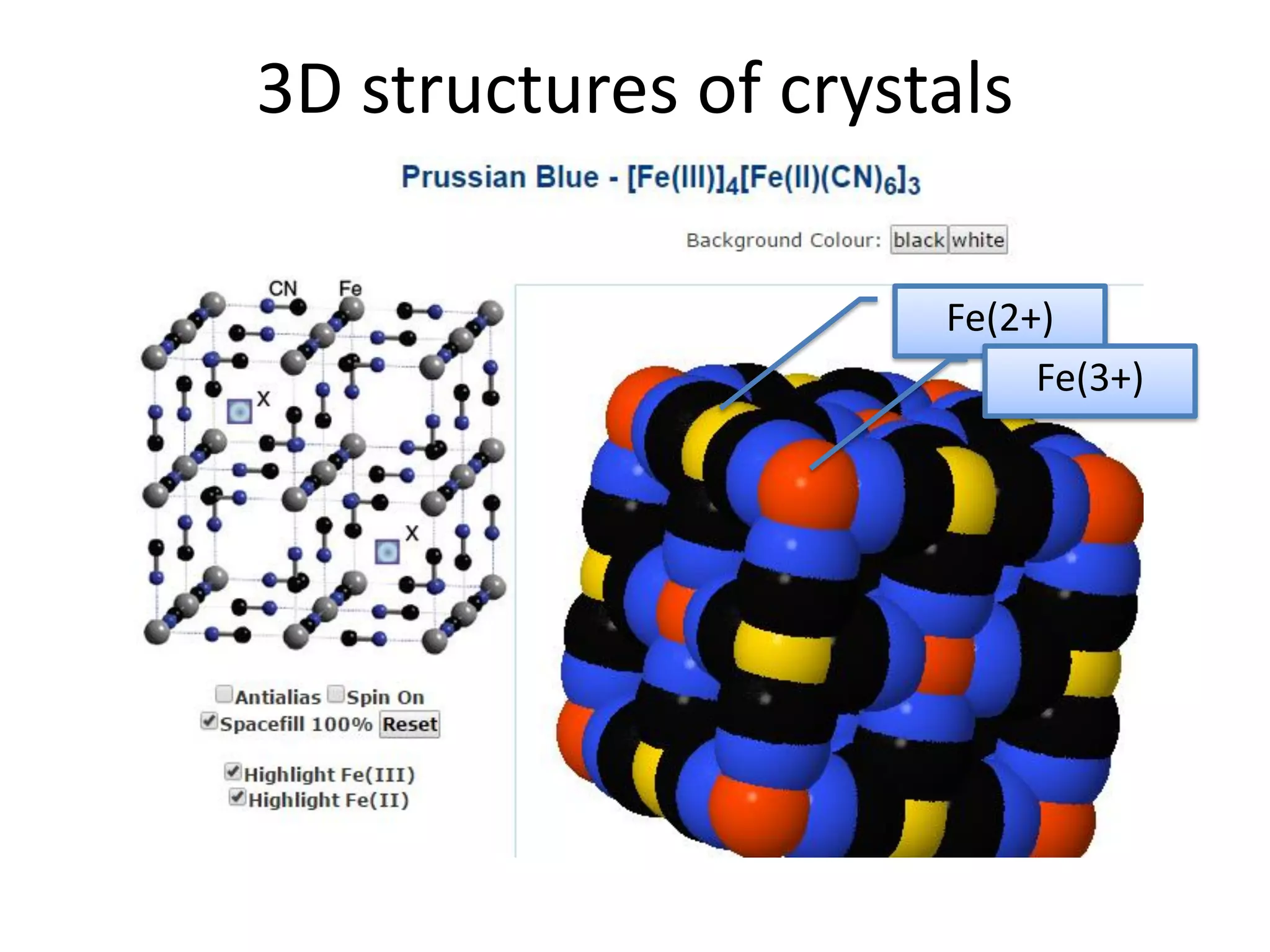
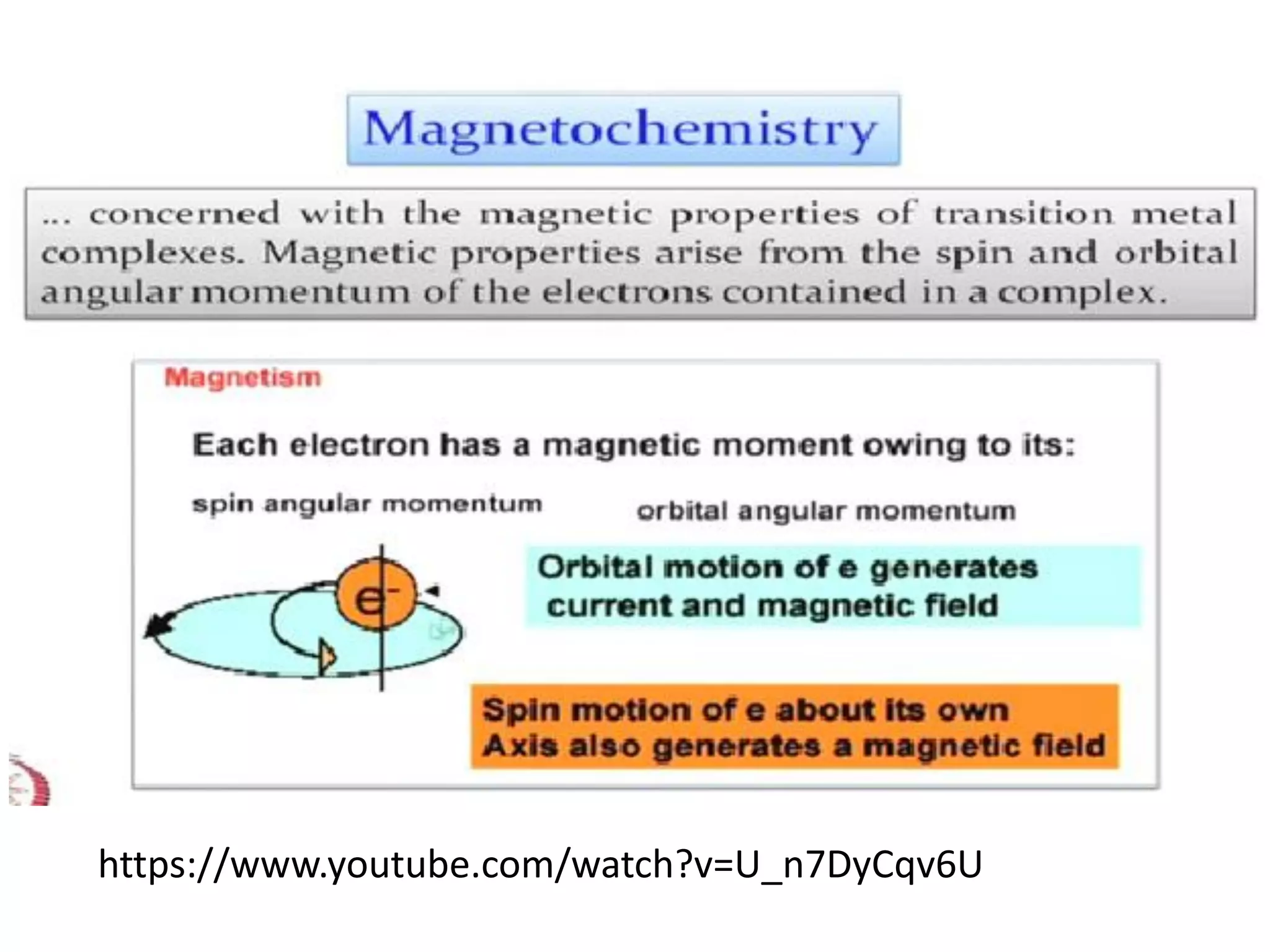
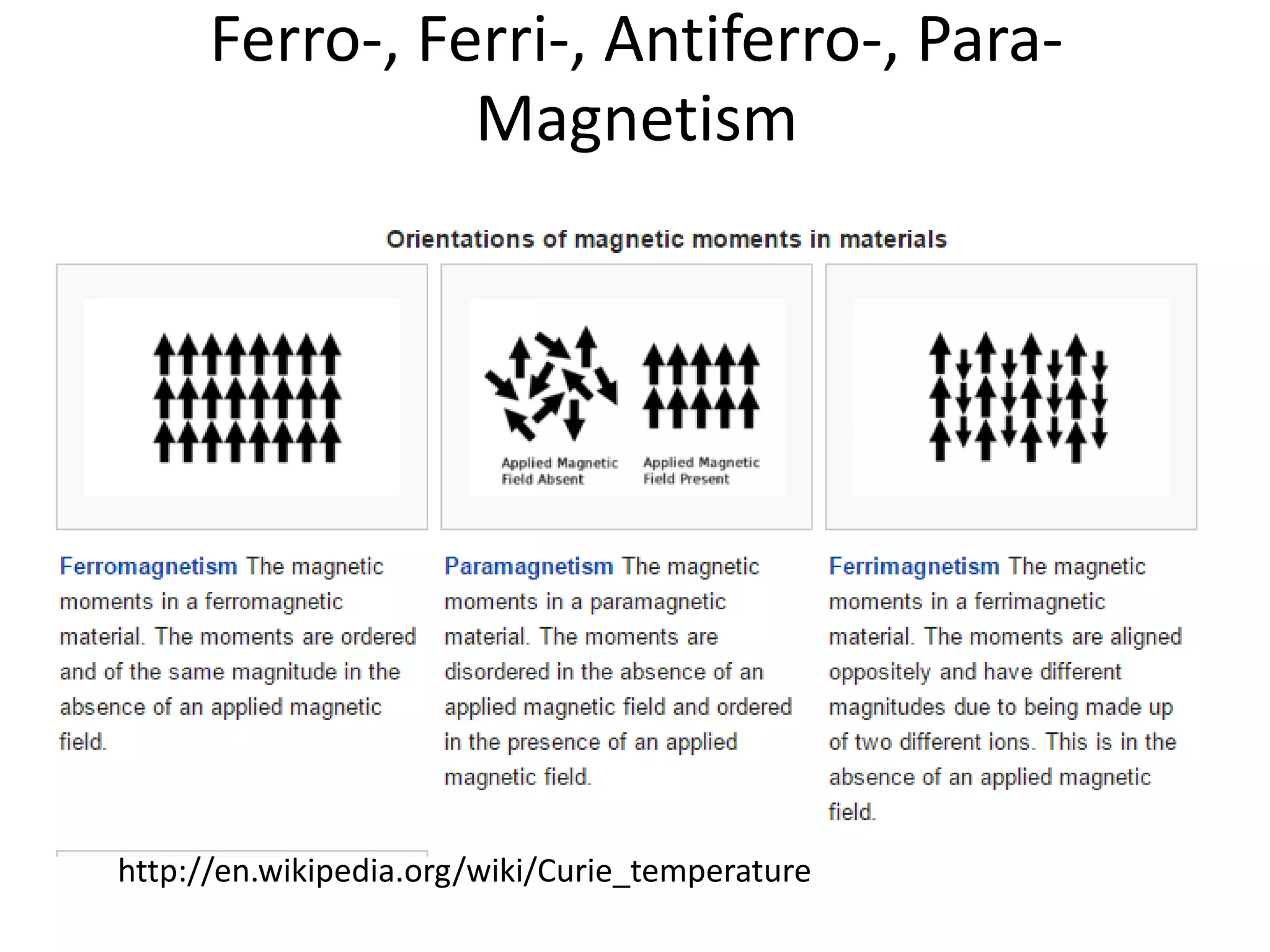
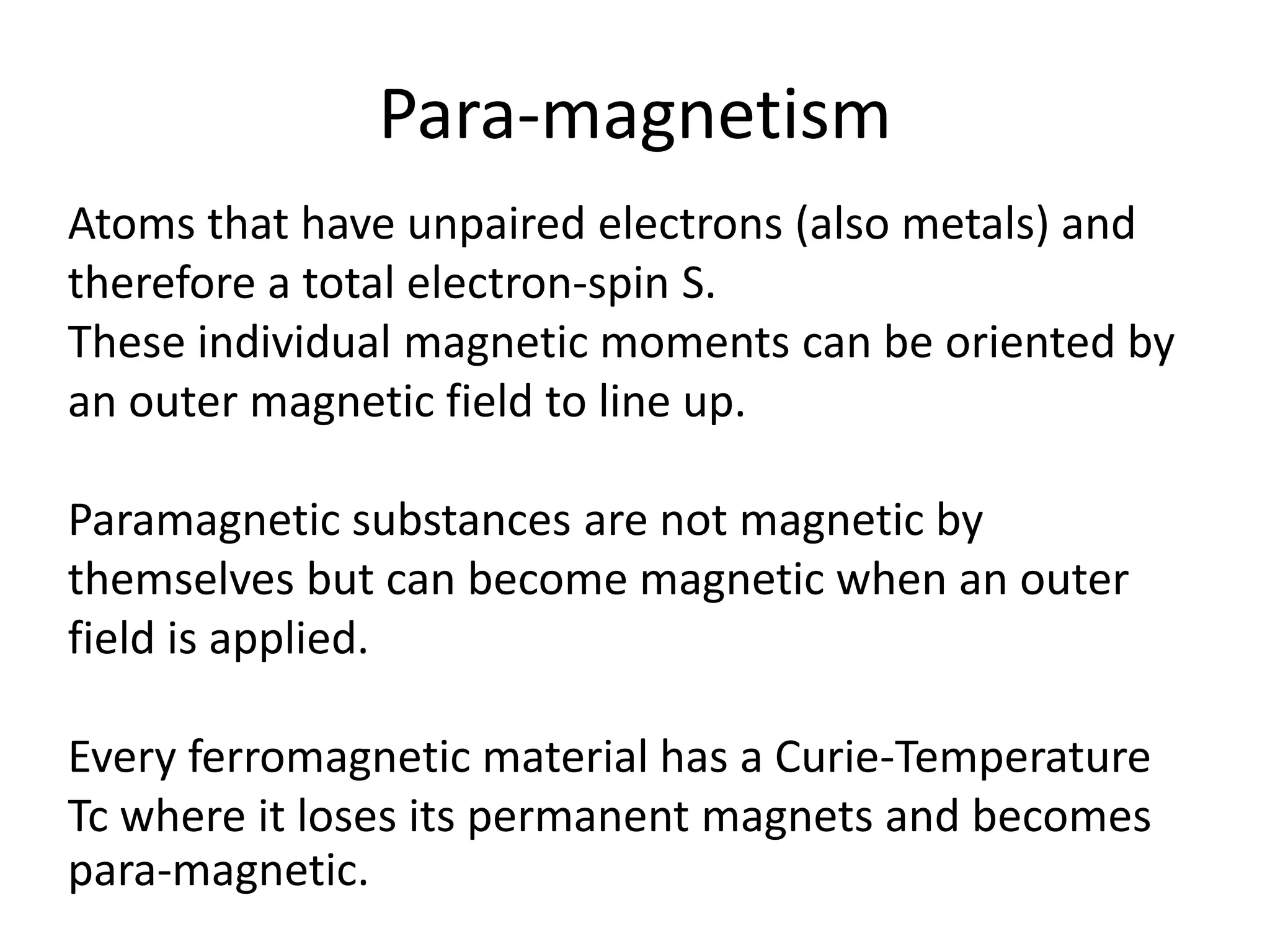
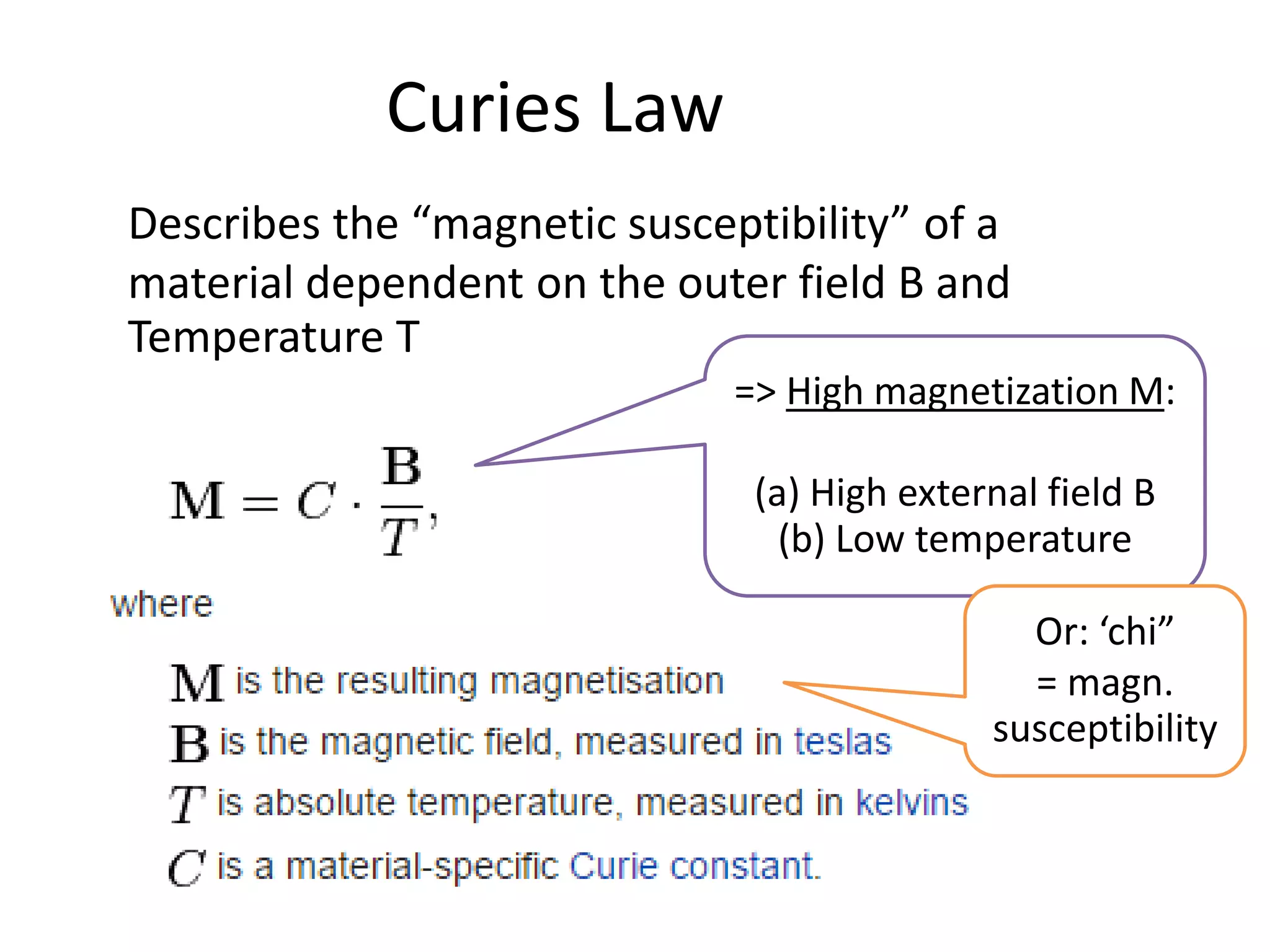
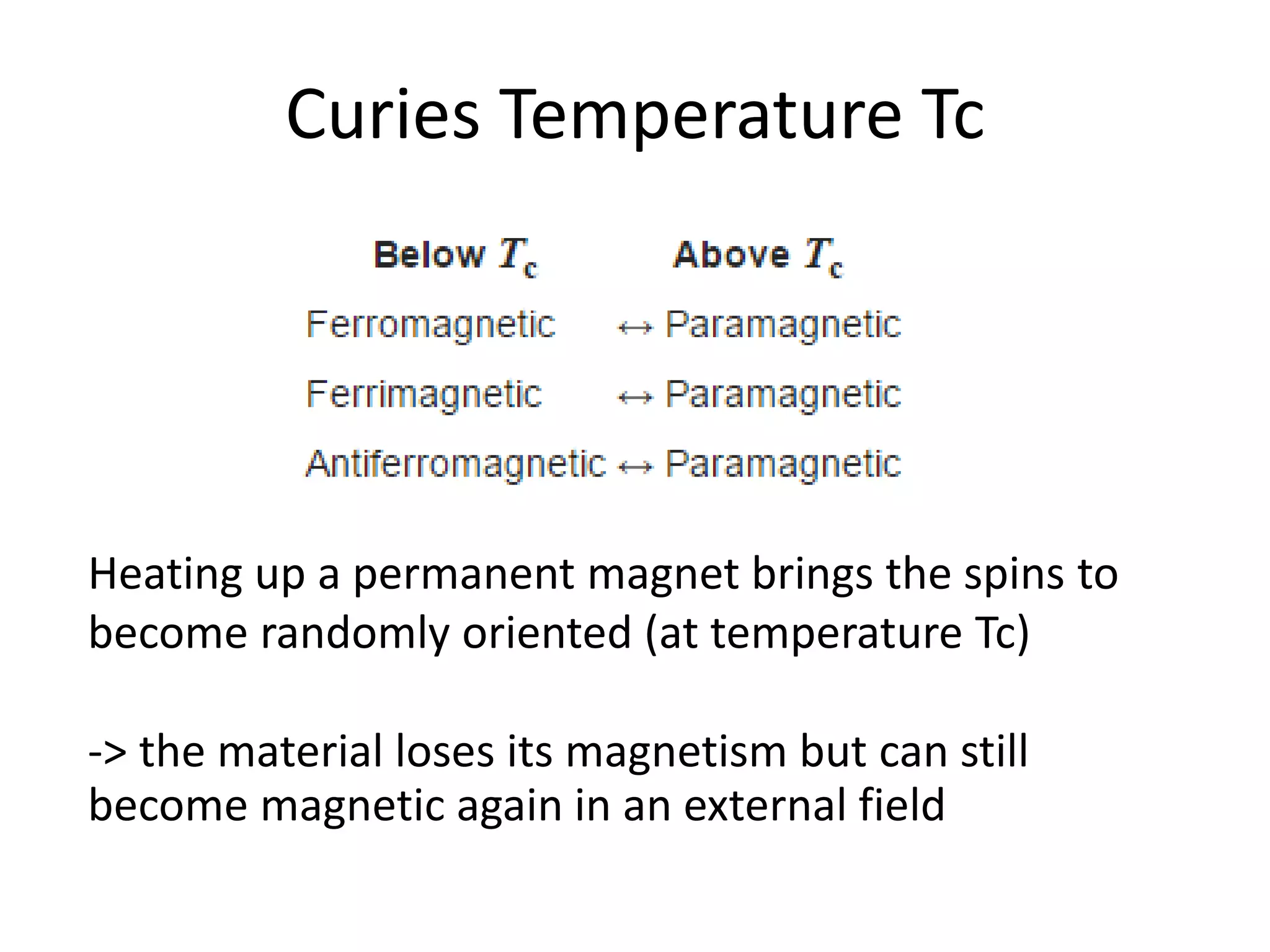
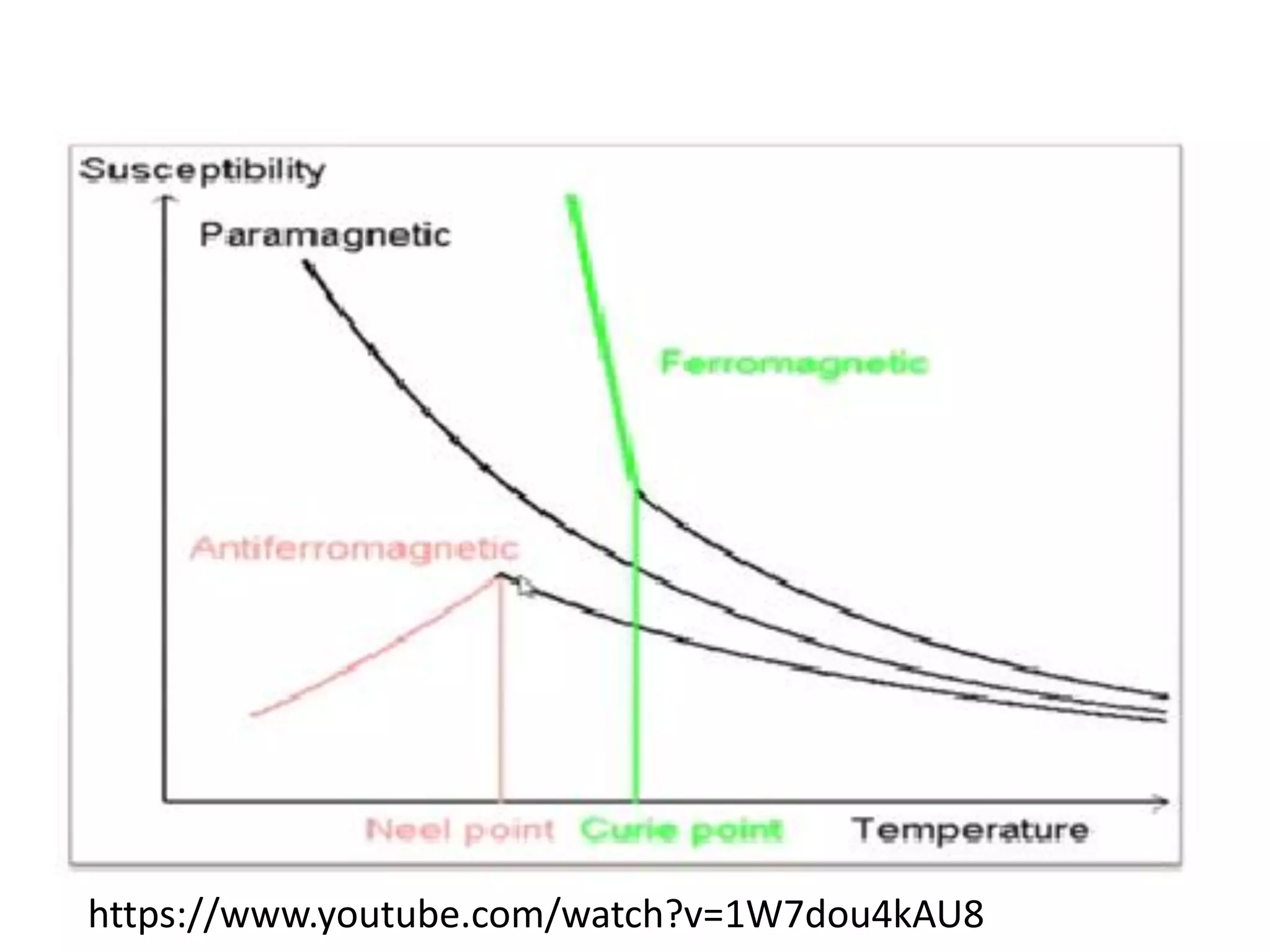
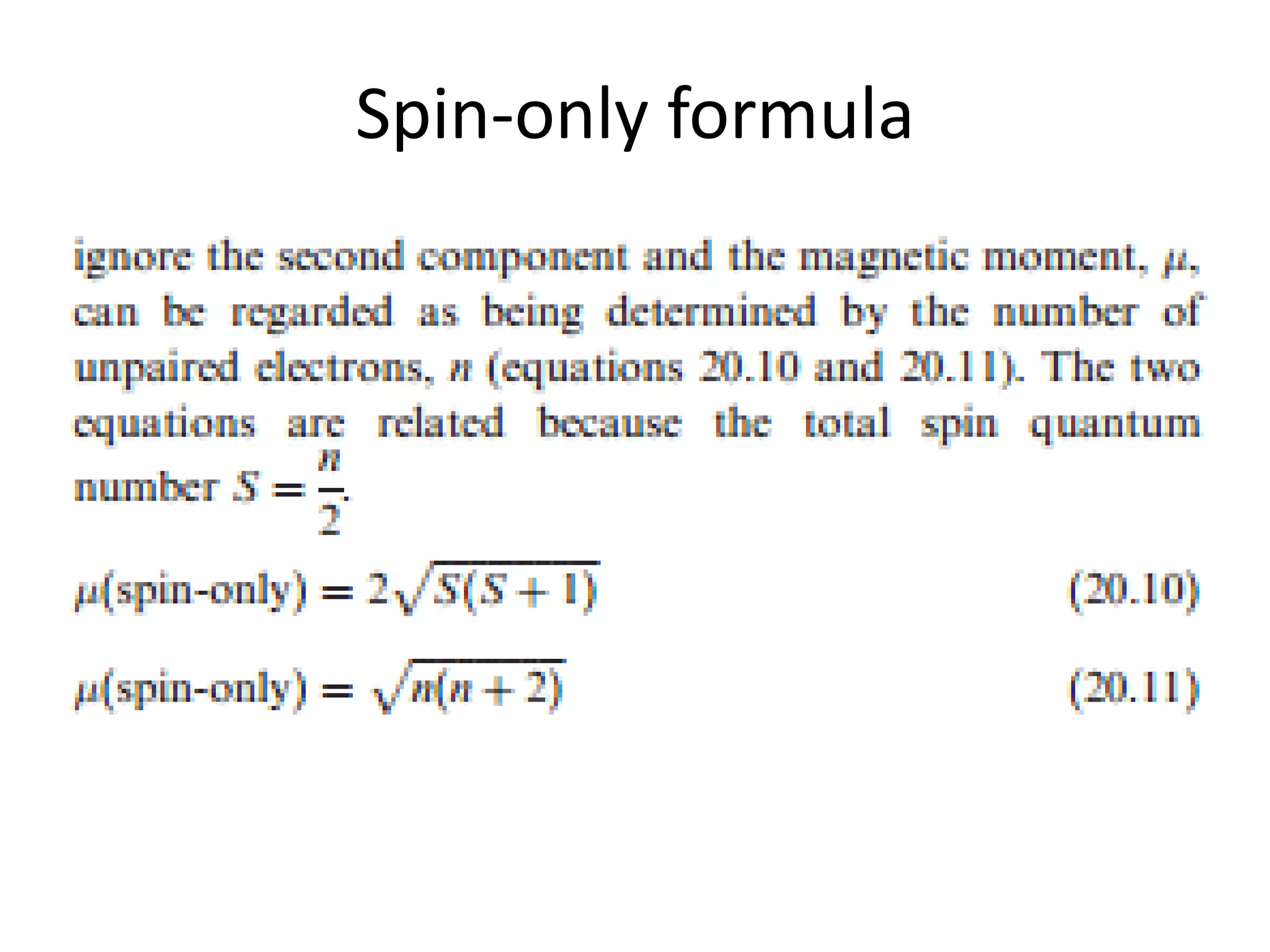
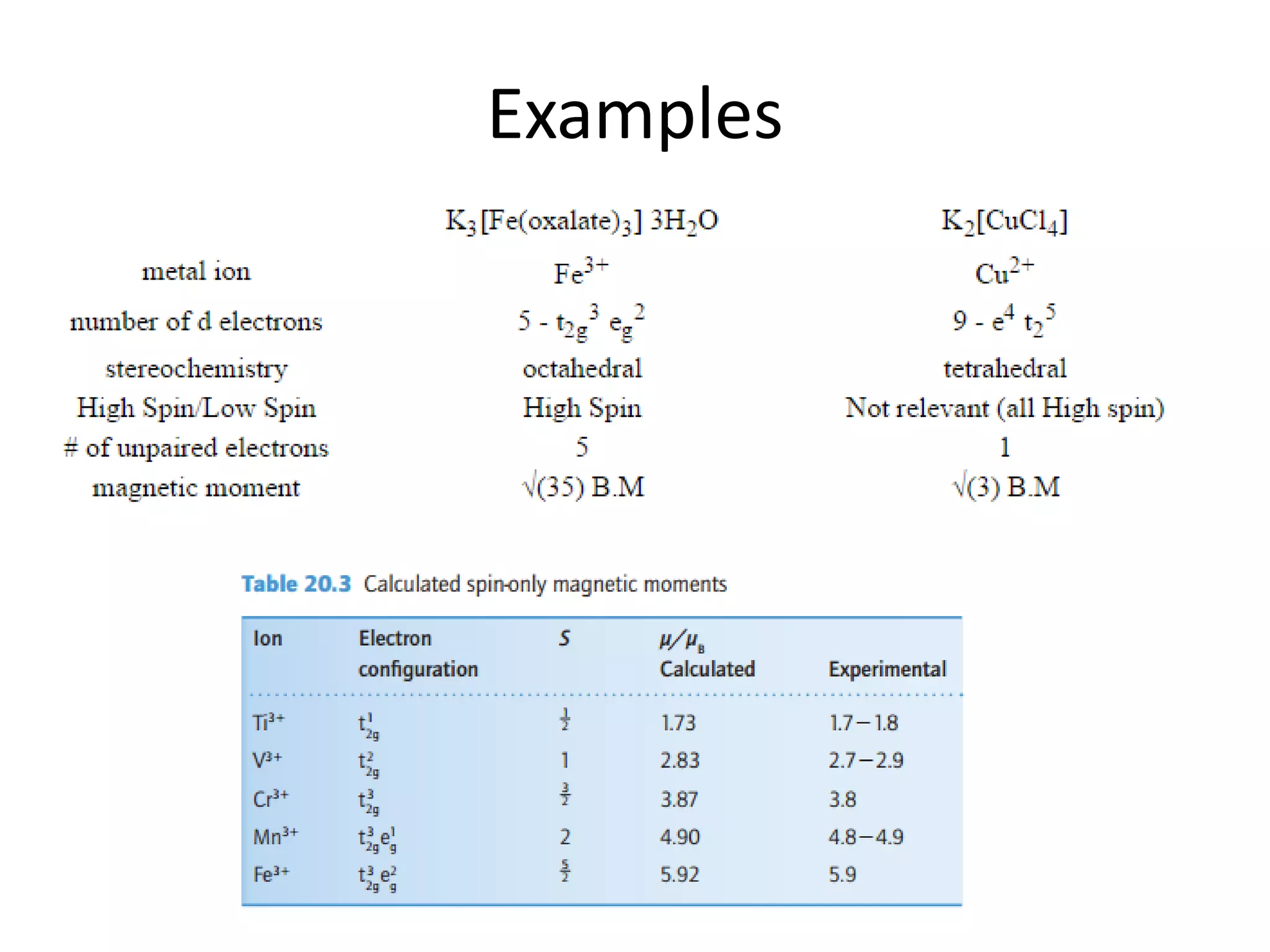
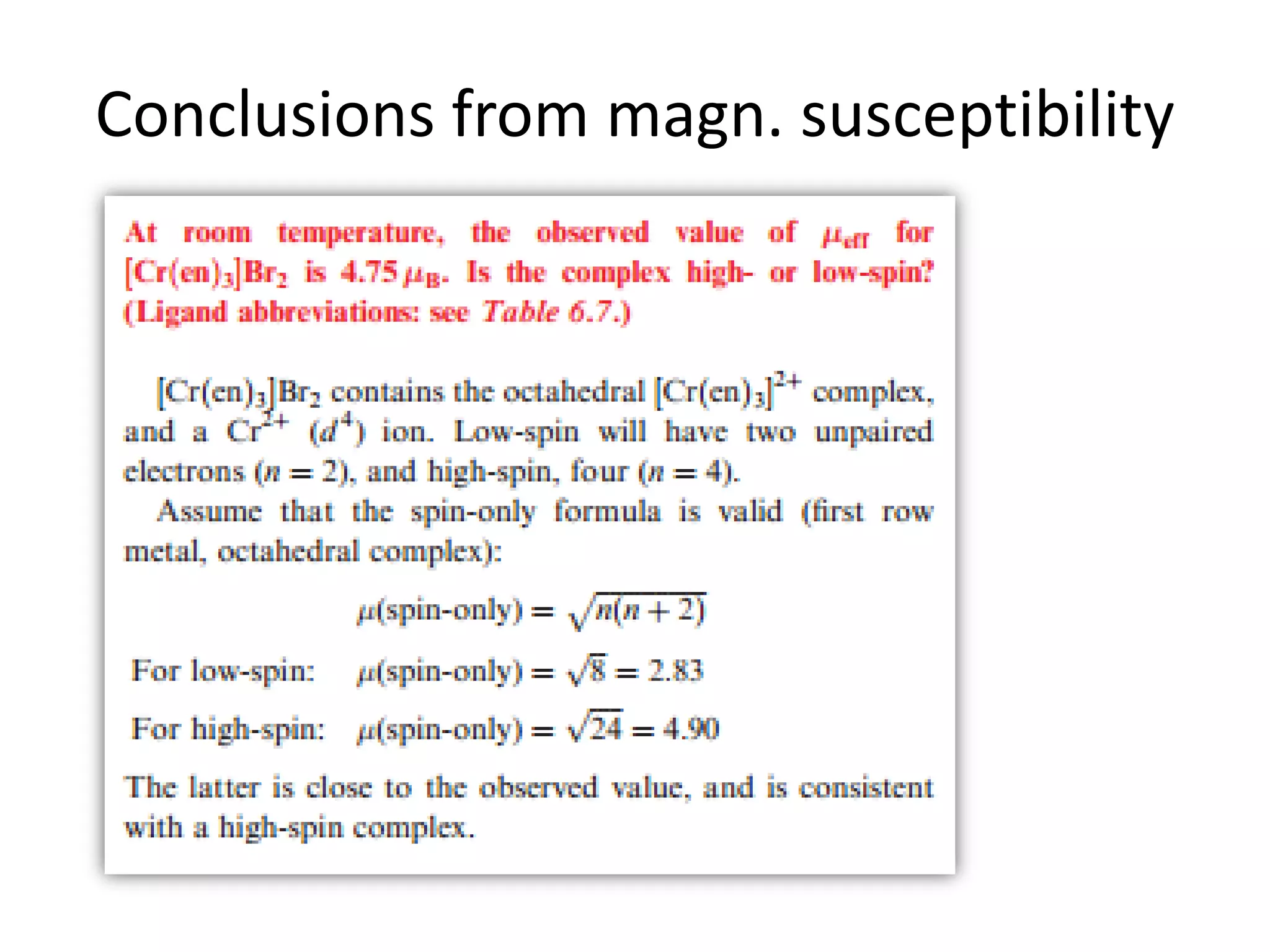
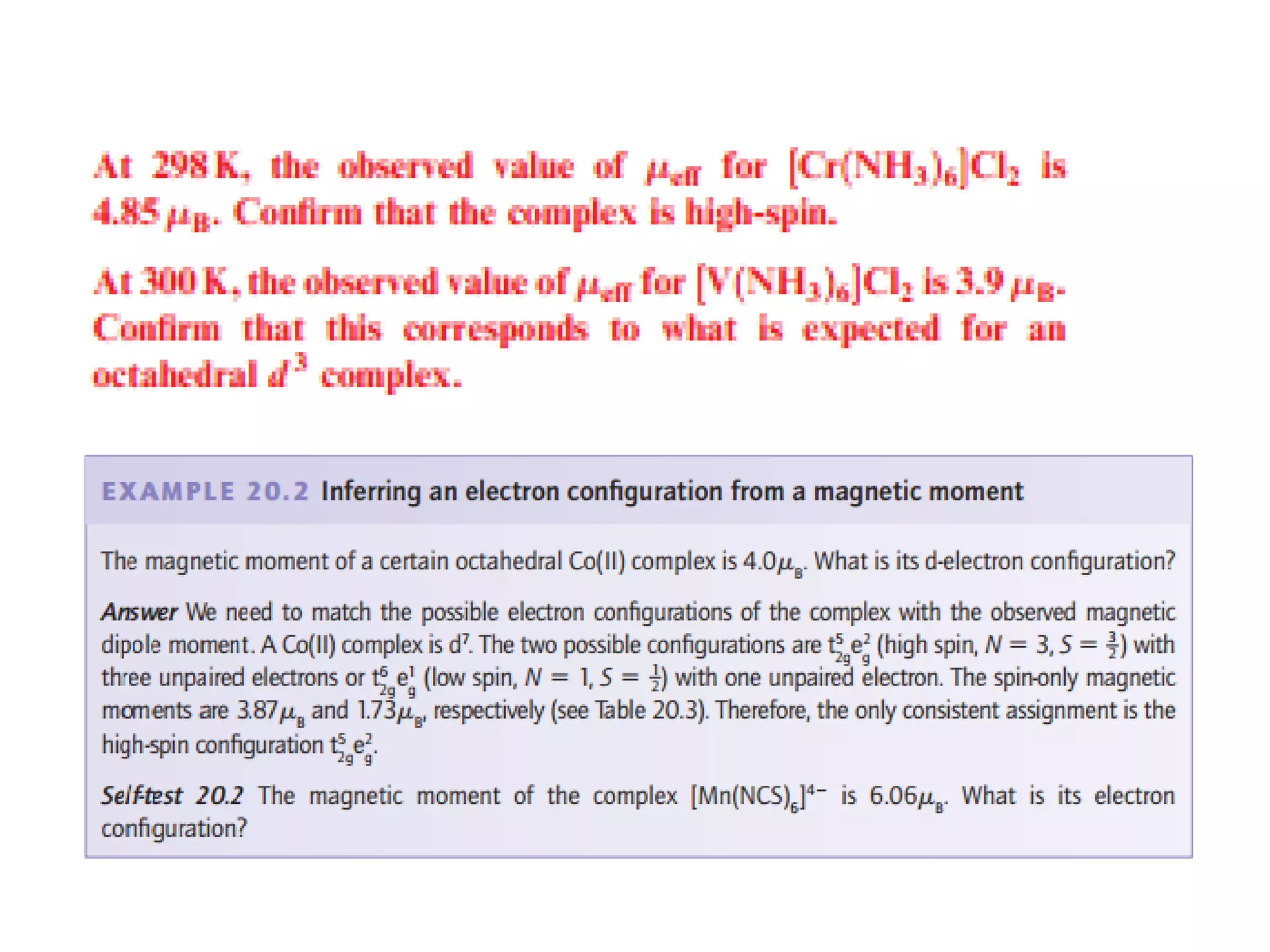
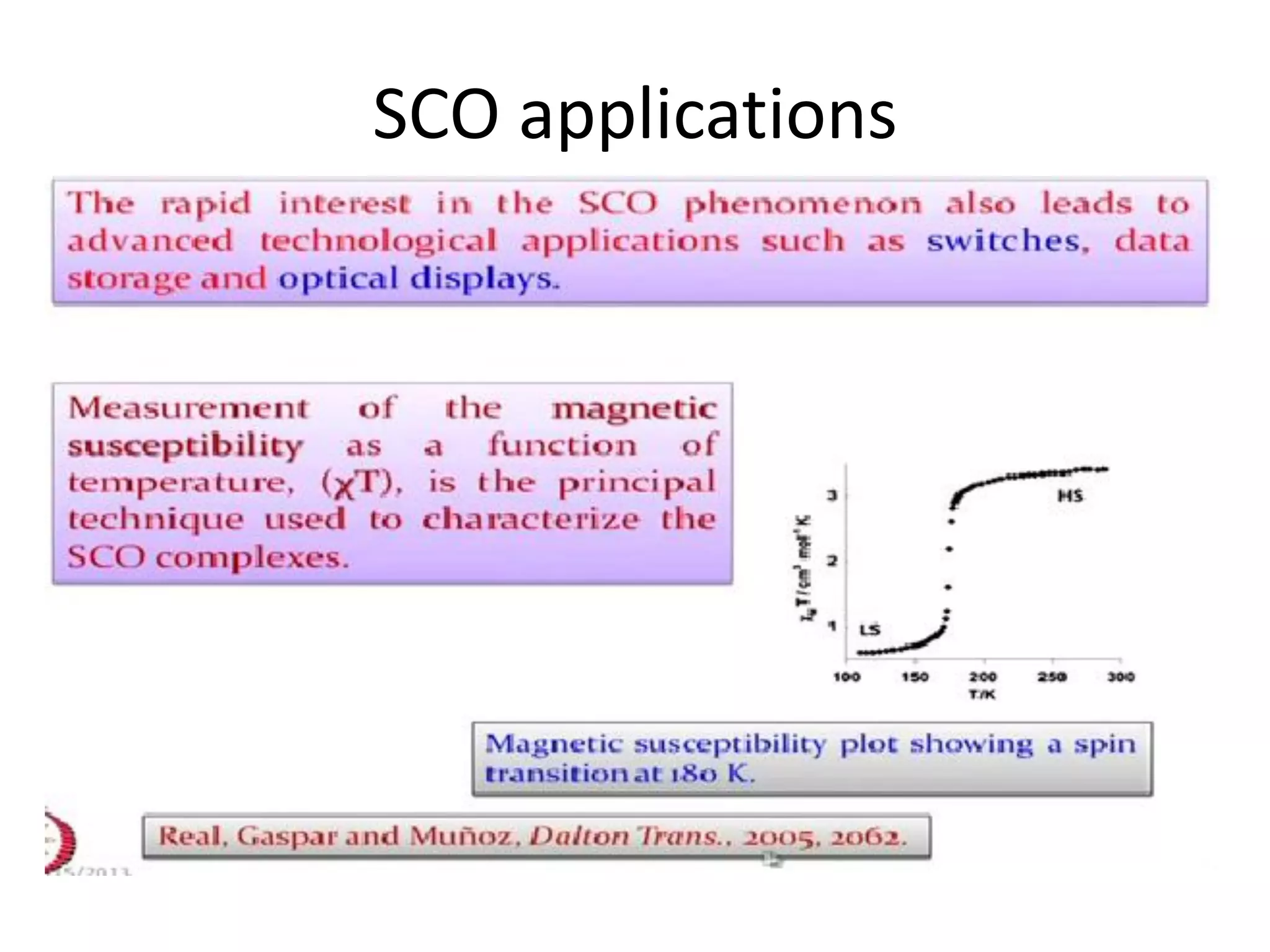
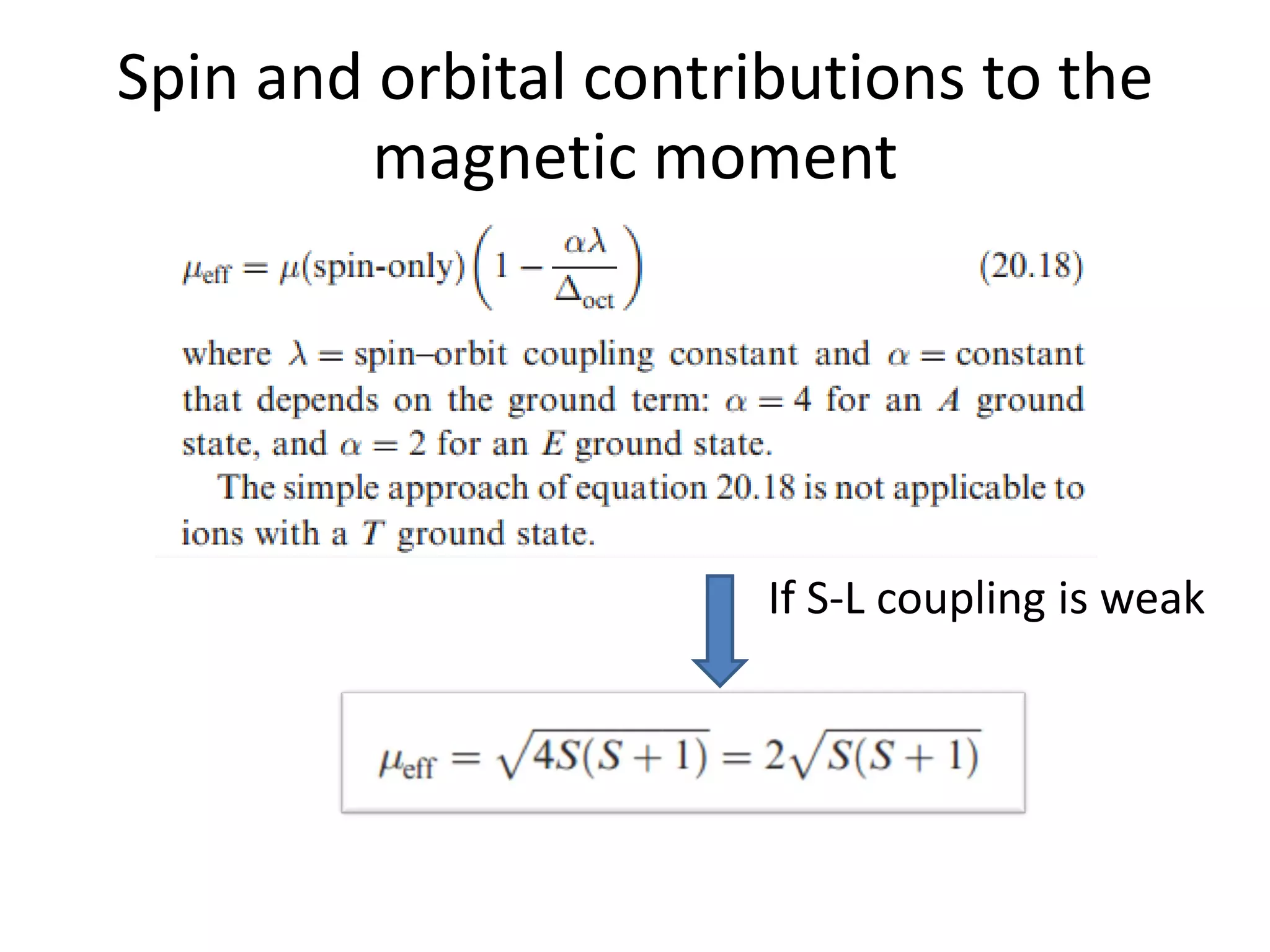
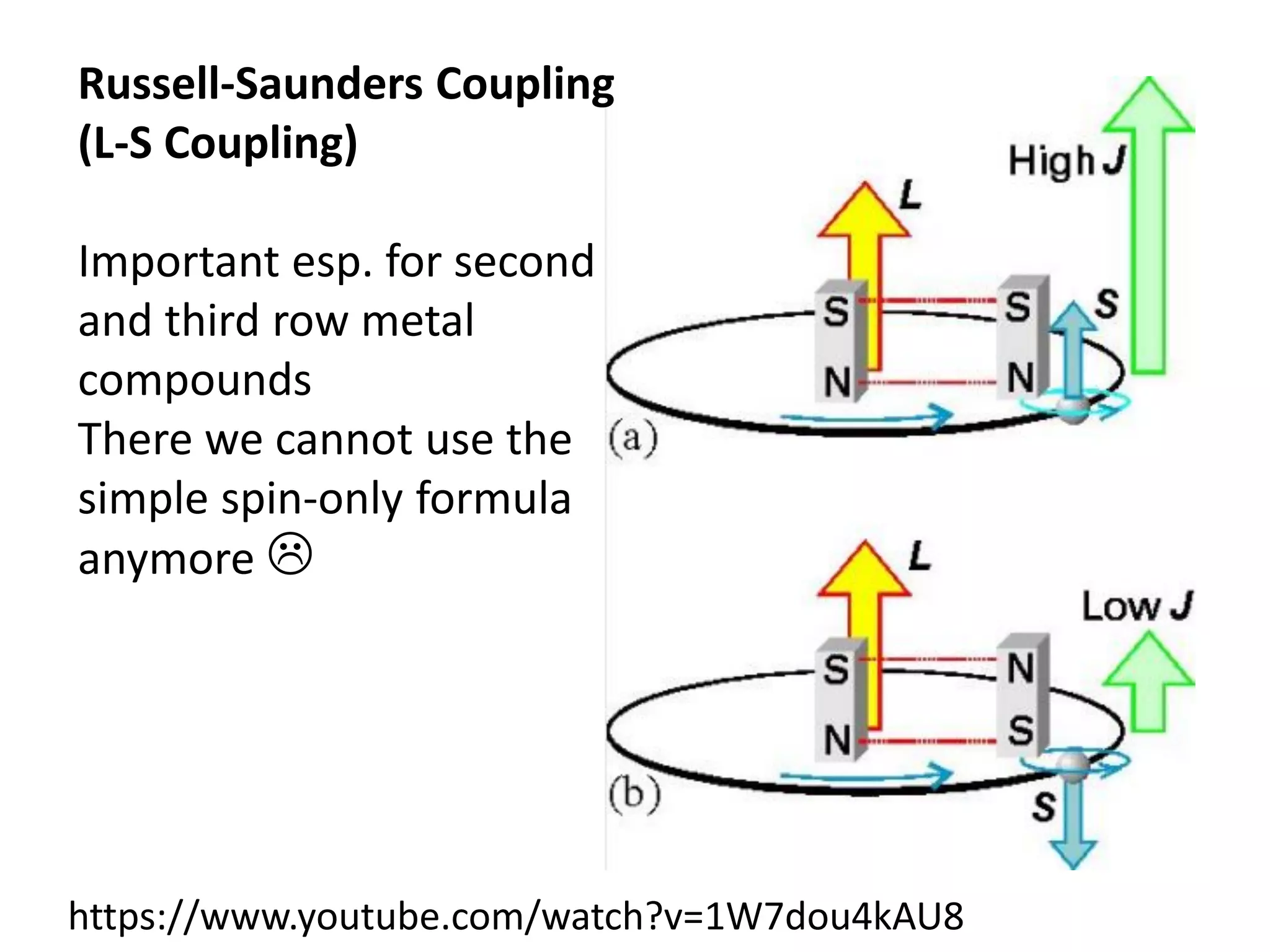
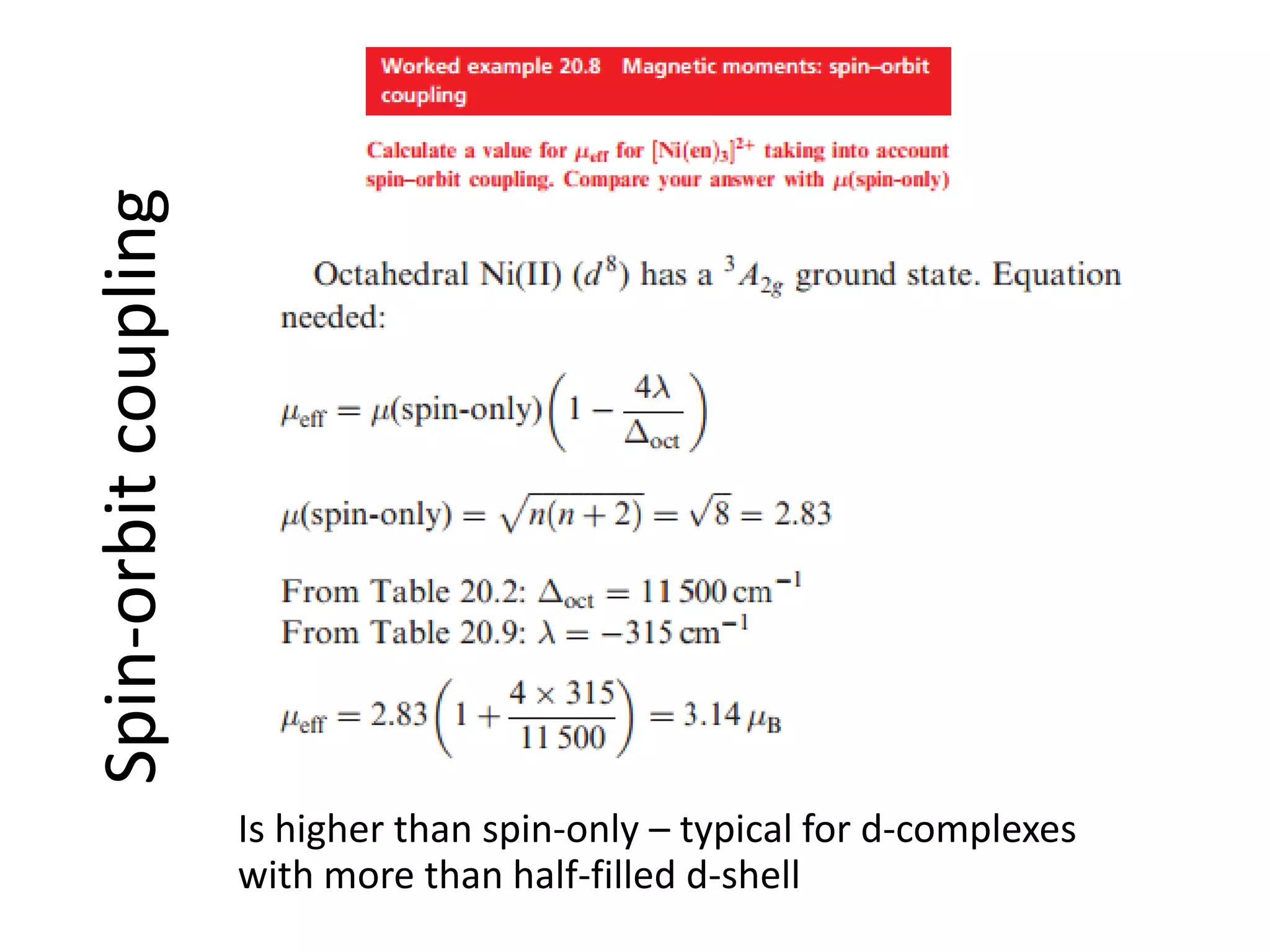
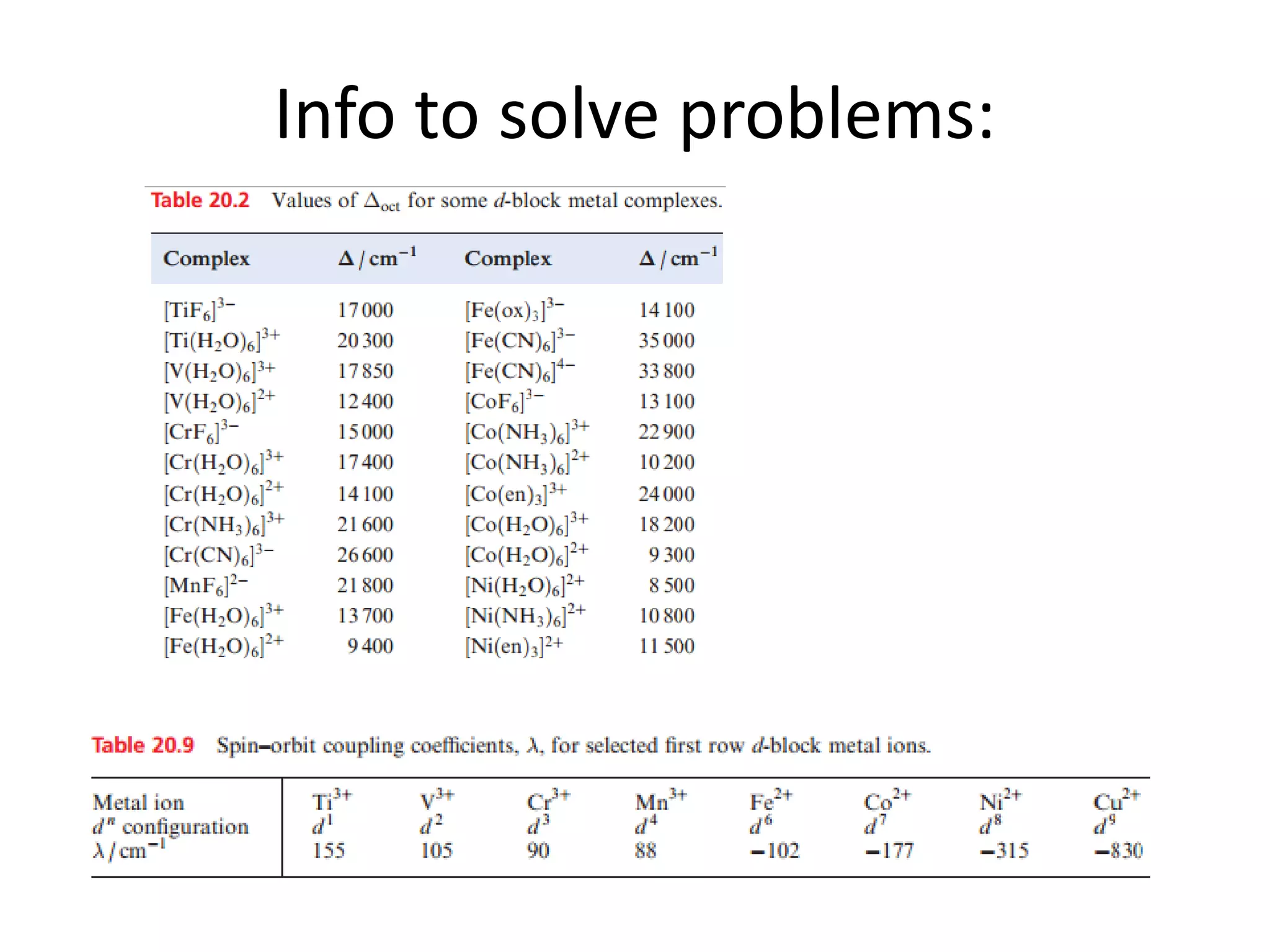
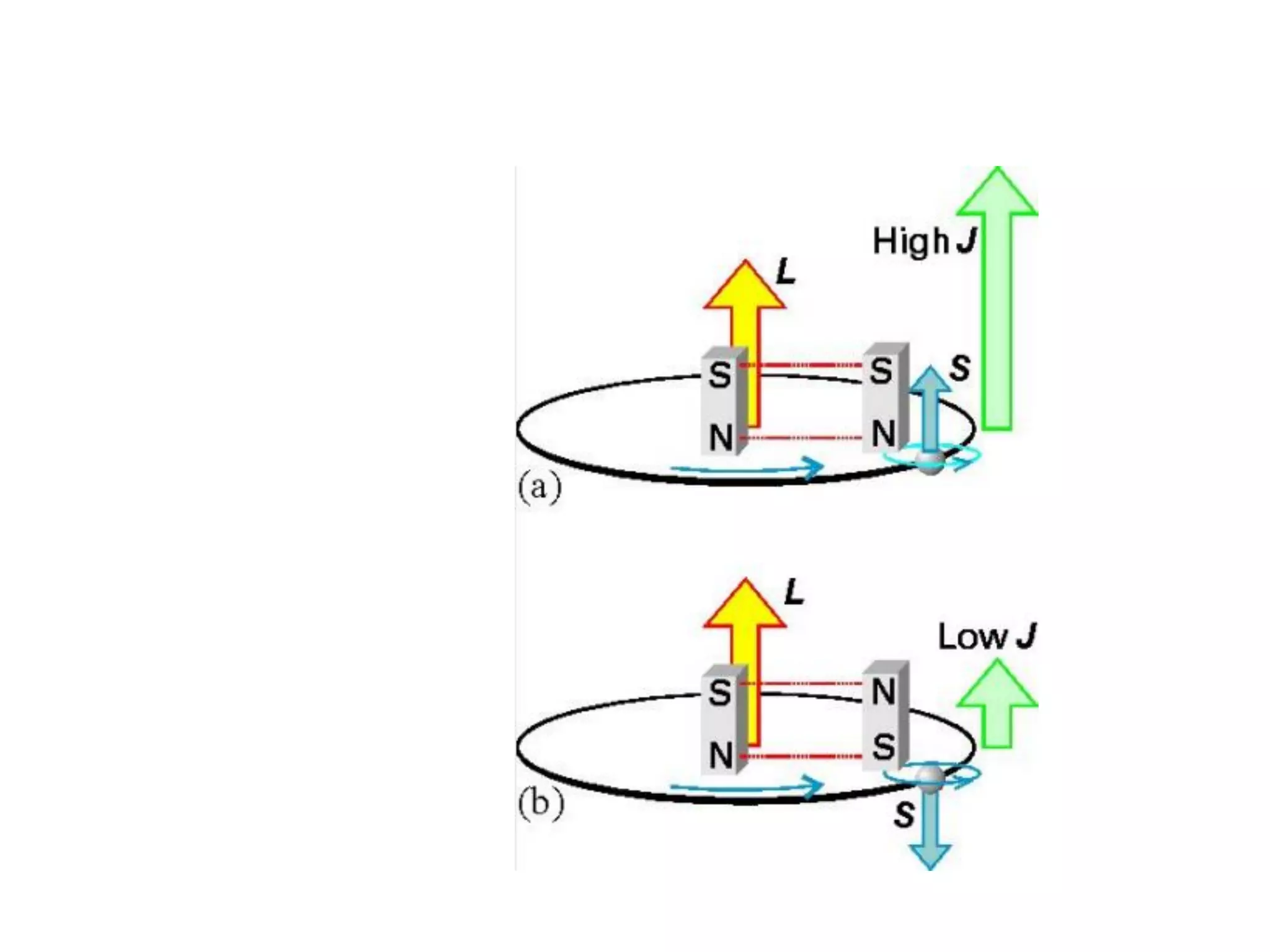
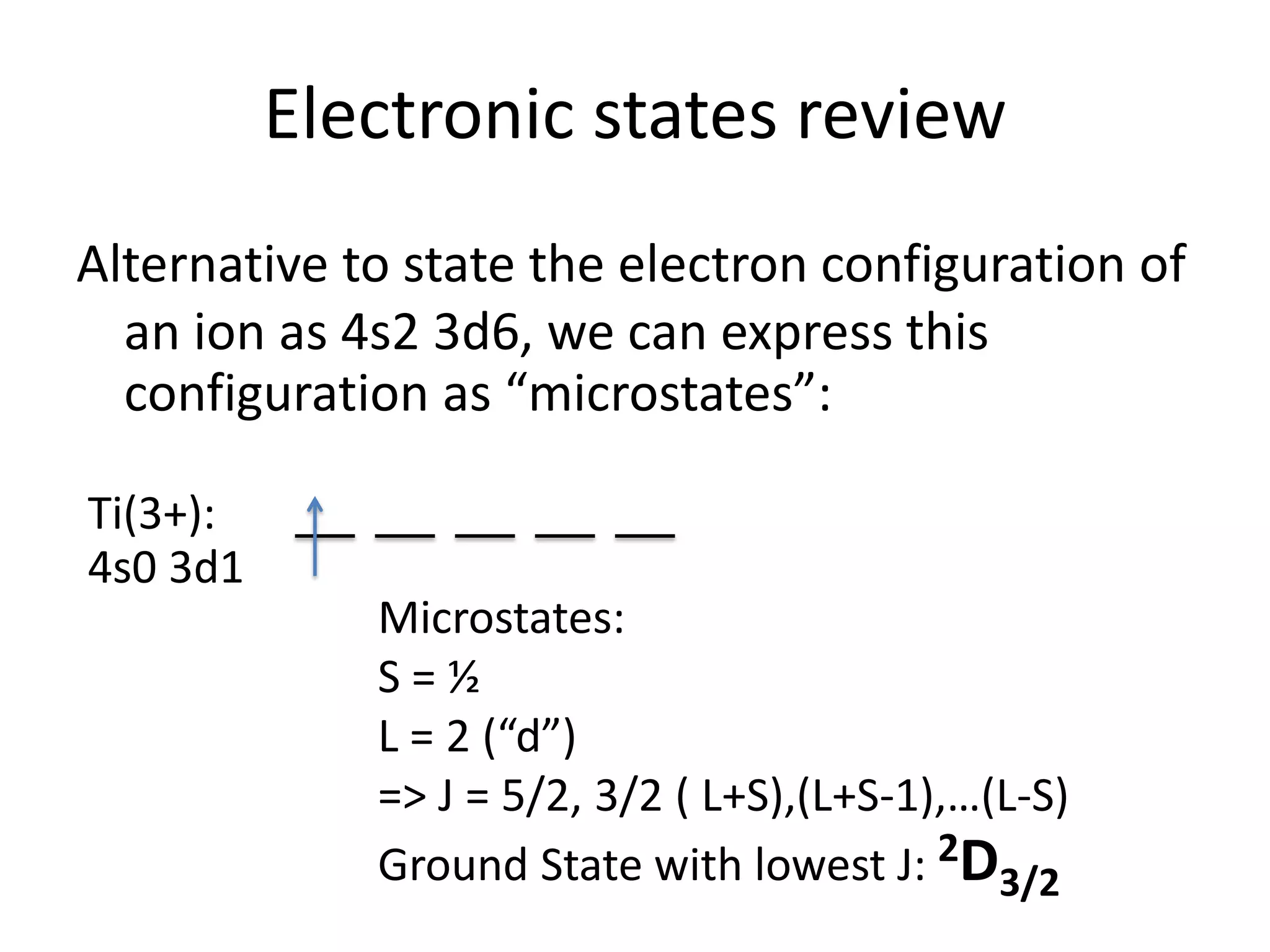
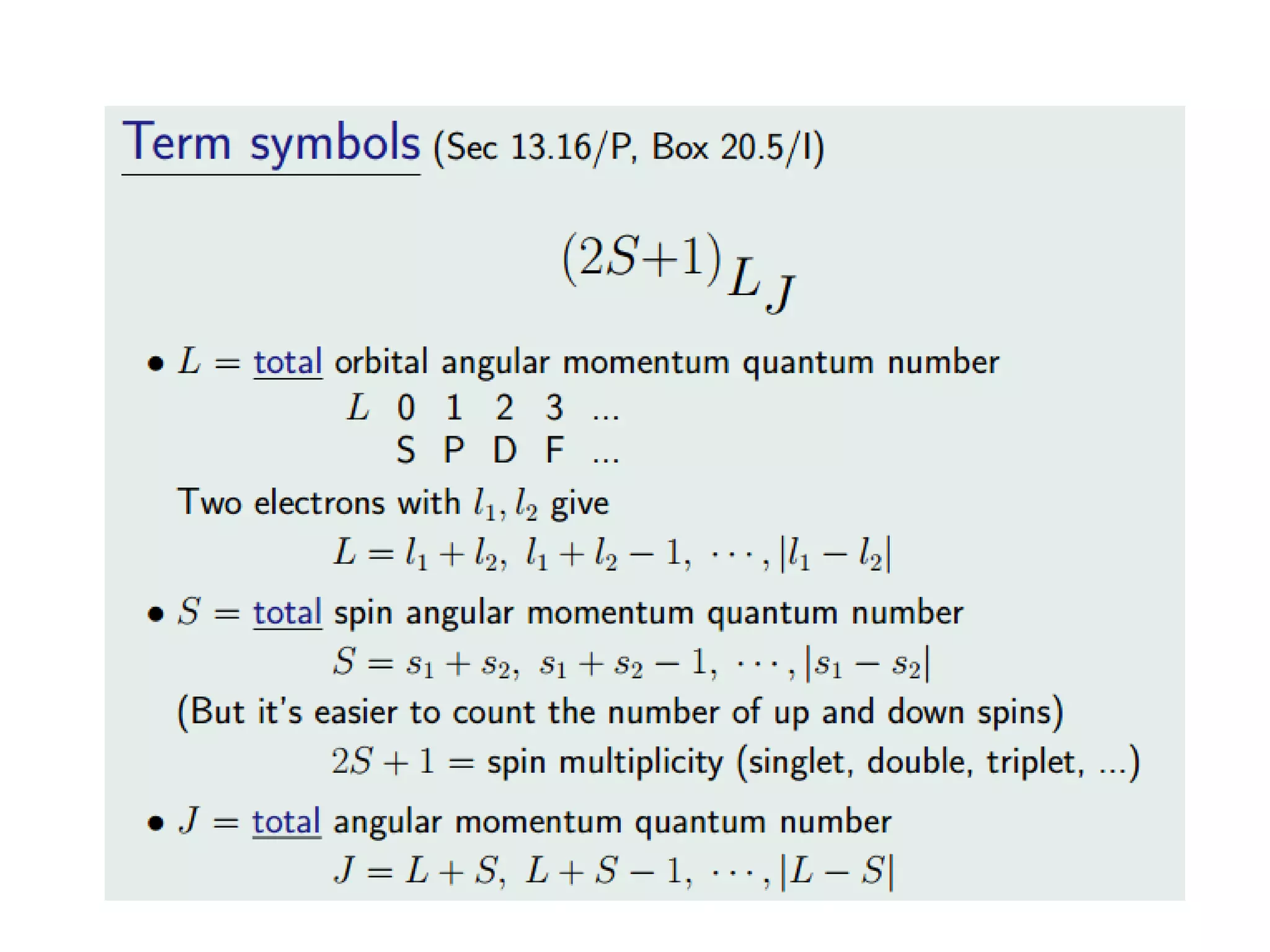
The document discusses the properties of coordination compounds, focusing on various examples and their behavior in precipitation reactions and crystal field theory applications. It covers topics such as electronic spectra, ligand field stabilization energy, and the stability of coordination compounds, including the impact of different ligands on stability and properties. Additionally, it delves into magnetism and the concepts underlying paramagnetism and spin crossover in coordination compounds.

![Example
A) [Cr(NH3)3Cl3]
B) [Cr(NH3)6]Cl3
C) Na3[Cr(CN)6]
D) Na3[CrCl6]
Which of these compounds will form
a precipitation with AgNO3 ?](https://image.slidesharecdn.com/properties-of-coordination-complexes-2014-160213161422/75/Properties-of-coordination-complexes-Complete-3-2048.jpg)



![(1) Find the configuration (as tx
2gey
g / ext2
y), the number of
unpaired electrons and the LFSE (in terms of ∆o / ∆t and P)
(2) Which of the following complexes has higher LFSE:
(h) [MnF6]3- i) [NiBr4]2- j) [Fe(CN)6]4-](https://image.slidesharecdn.com/properties-of-coordination-complexes-2014-160213161422/75/Properties-of-coordination-complexes-Complete-15-2048.jpg)


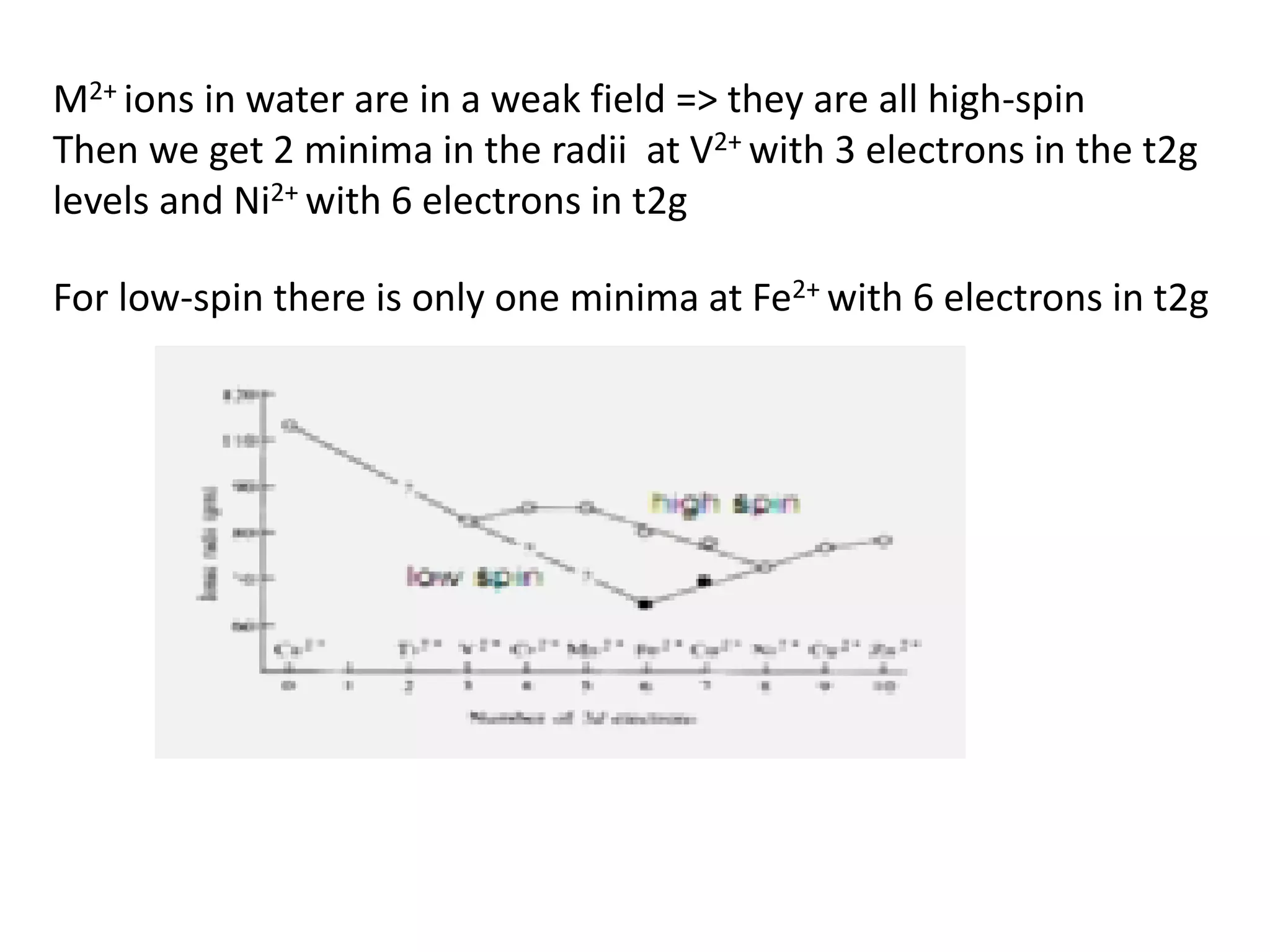
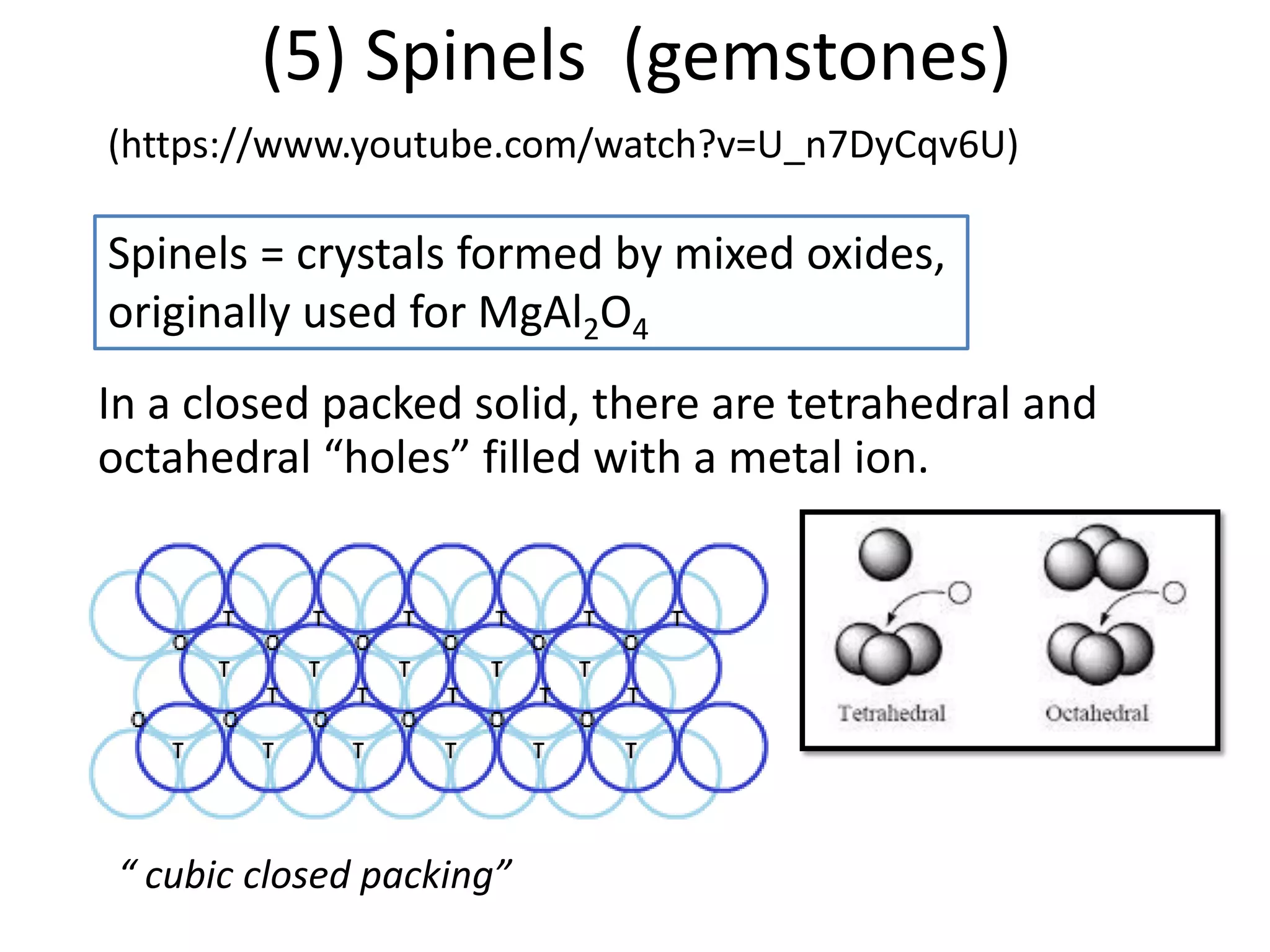

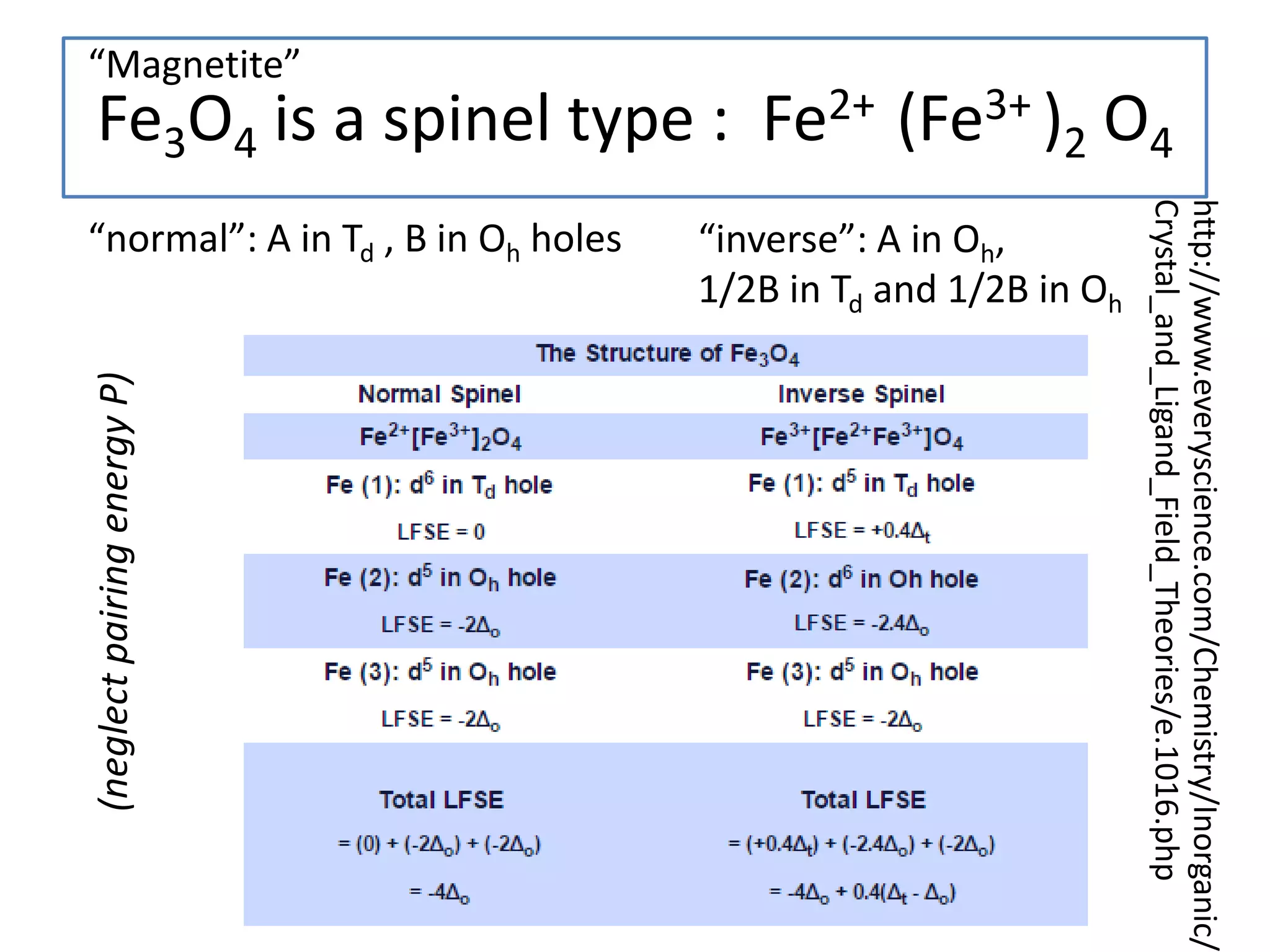
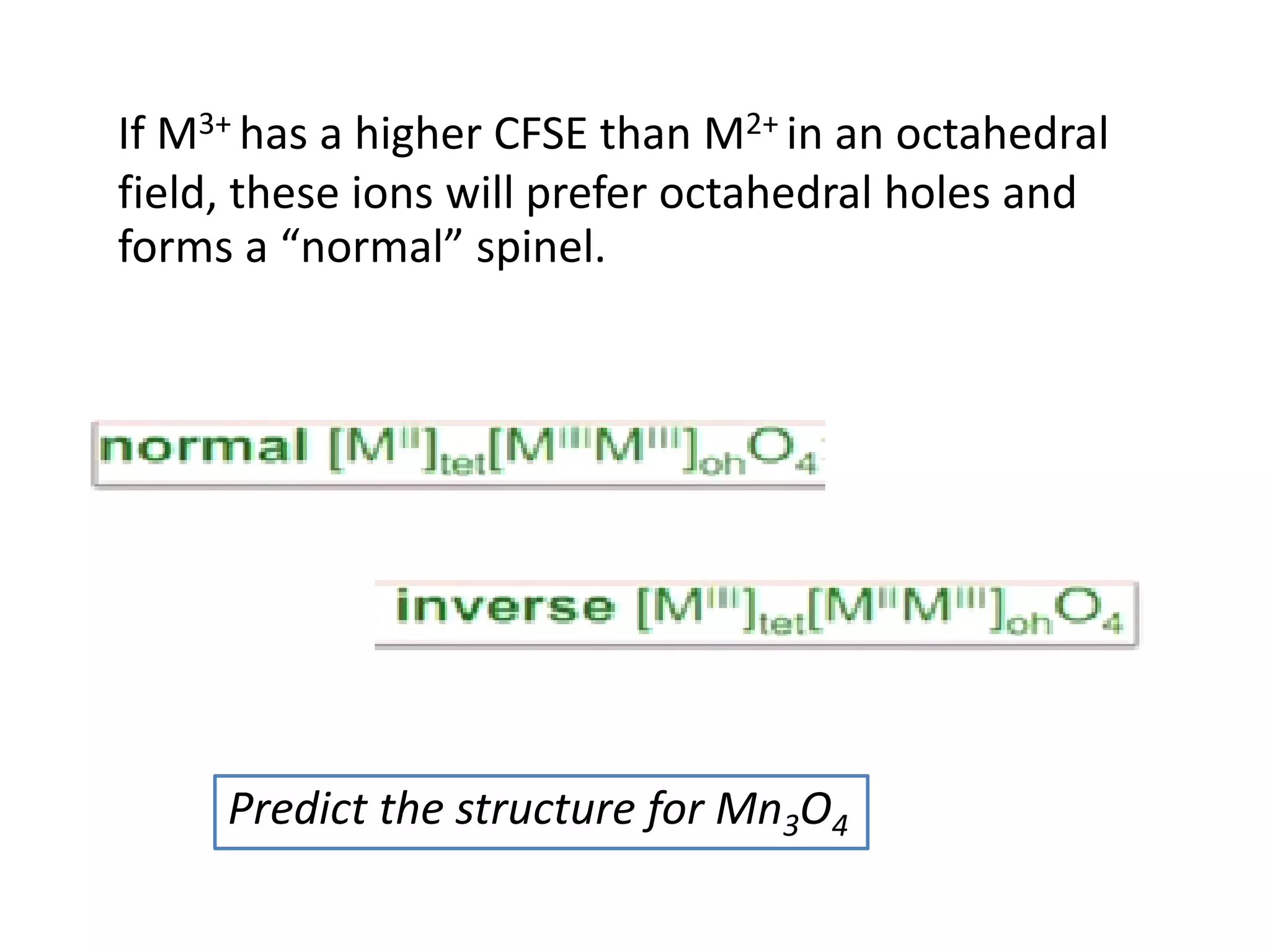




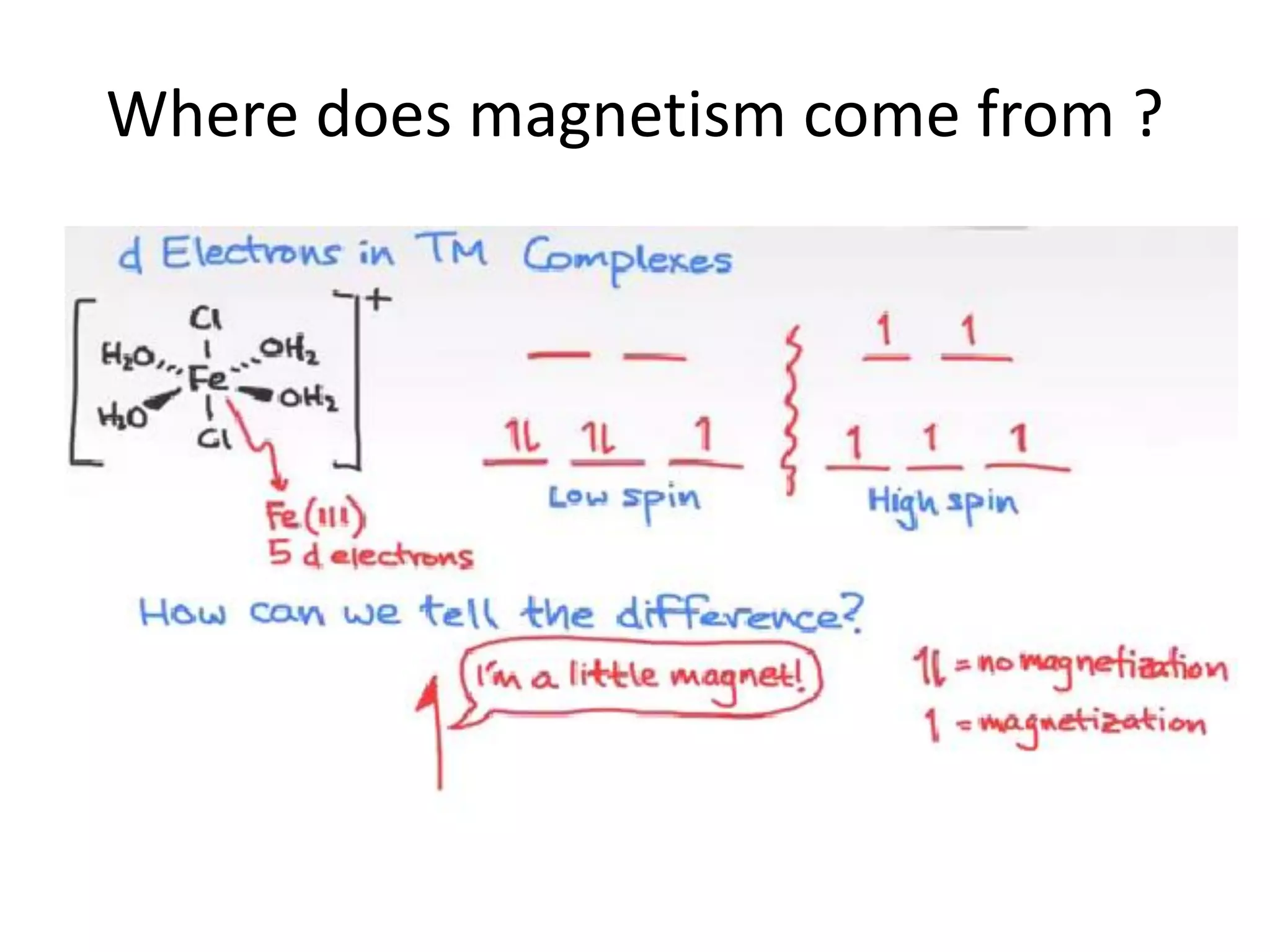
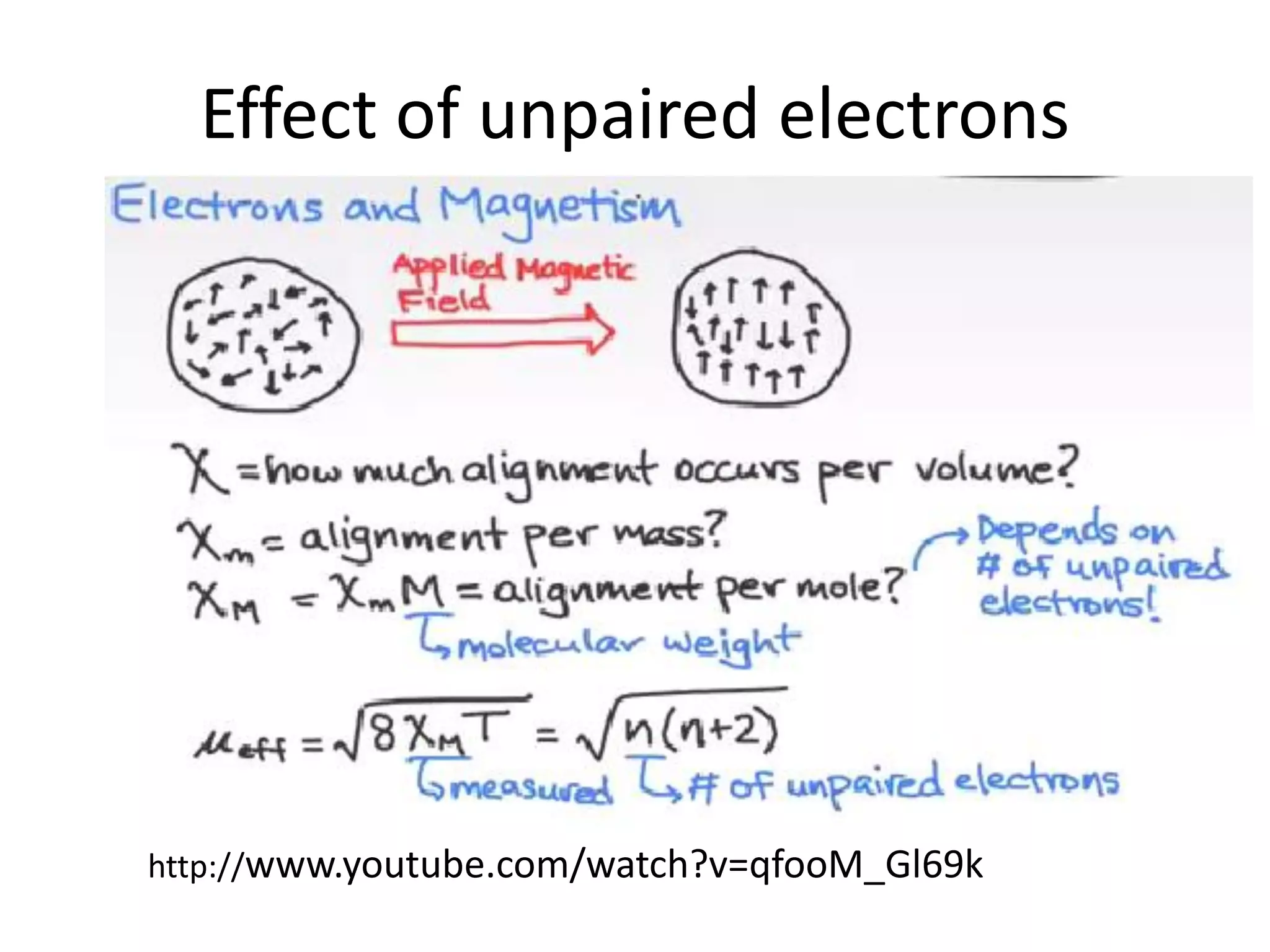
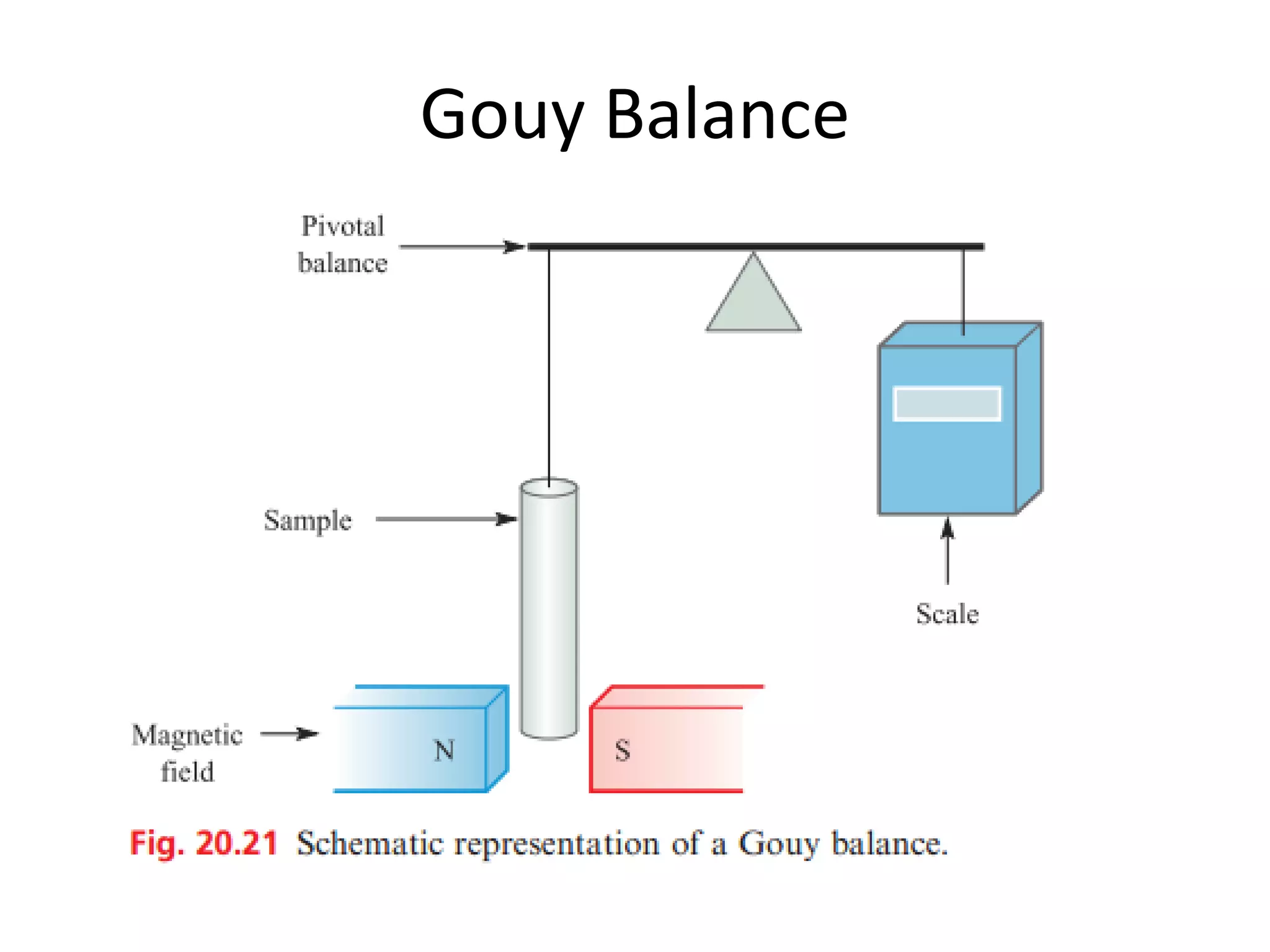
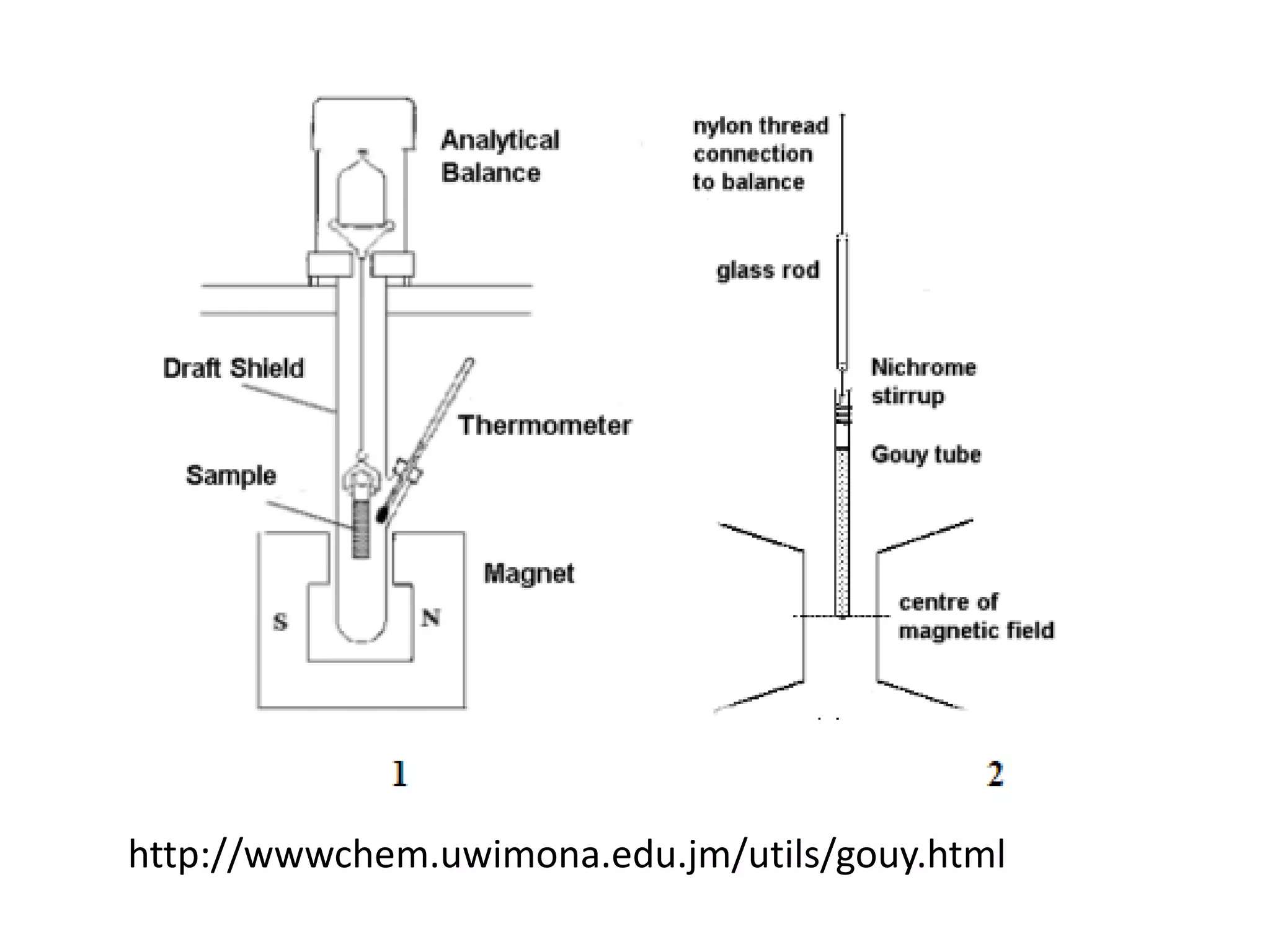

![Deviations from spin-only formula
Example:
[Fe(CN)6]3- has μ = 2.3μB
which is between low- and high-spin calculated
(check this out)](https://image.slidesharecdn.com/properties-of-coordination-complexes-2014-160213161422/75/Properties-of-coordination-complexes-Complete-57-2048.jpg)



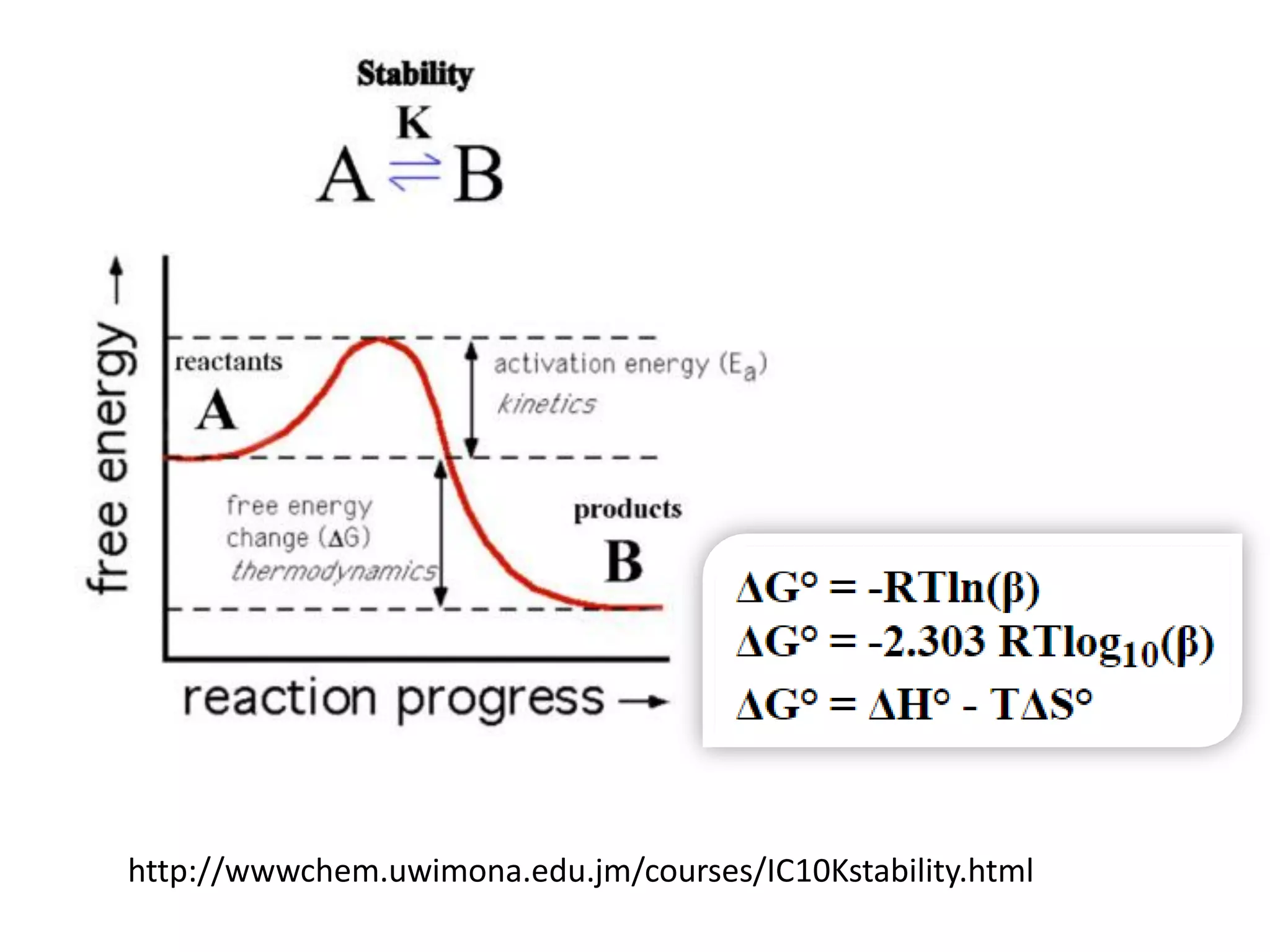
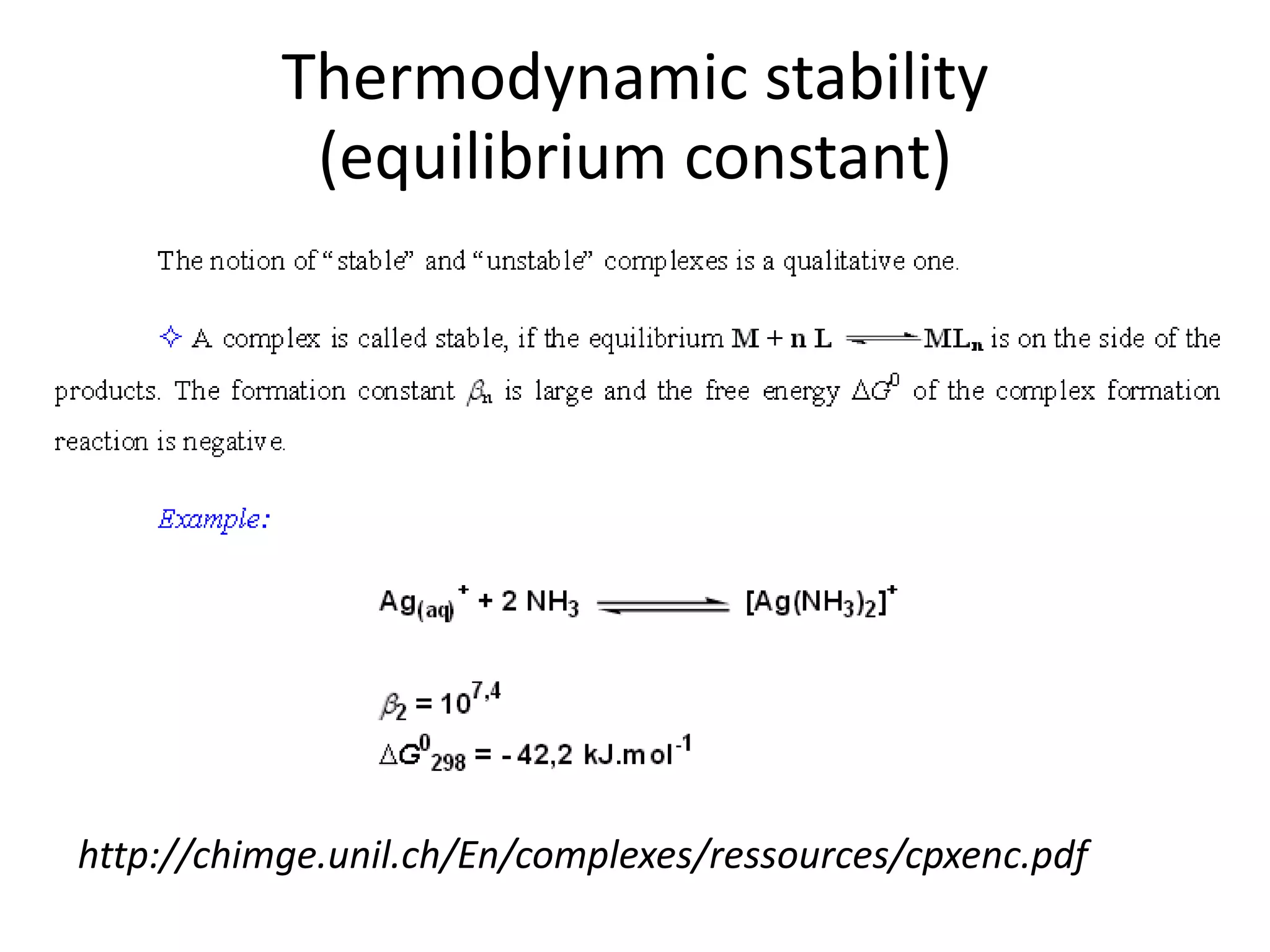
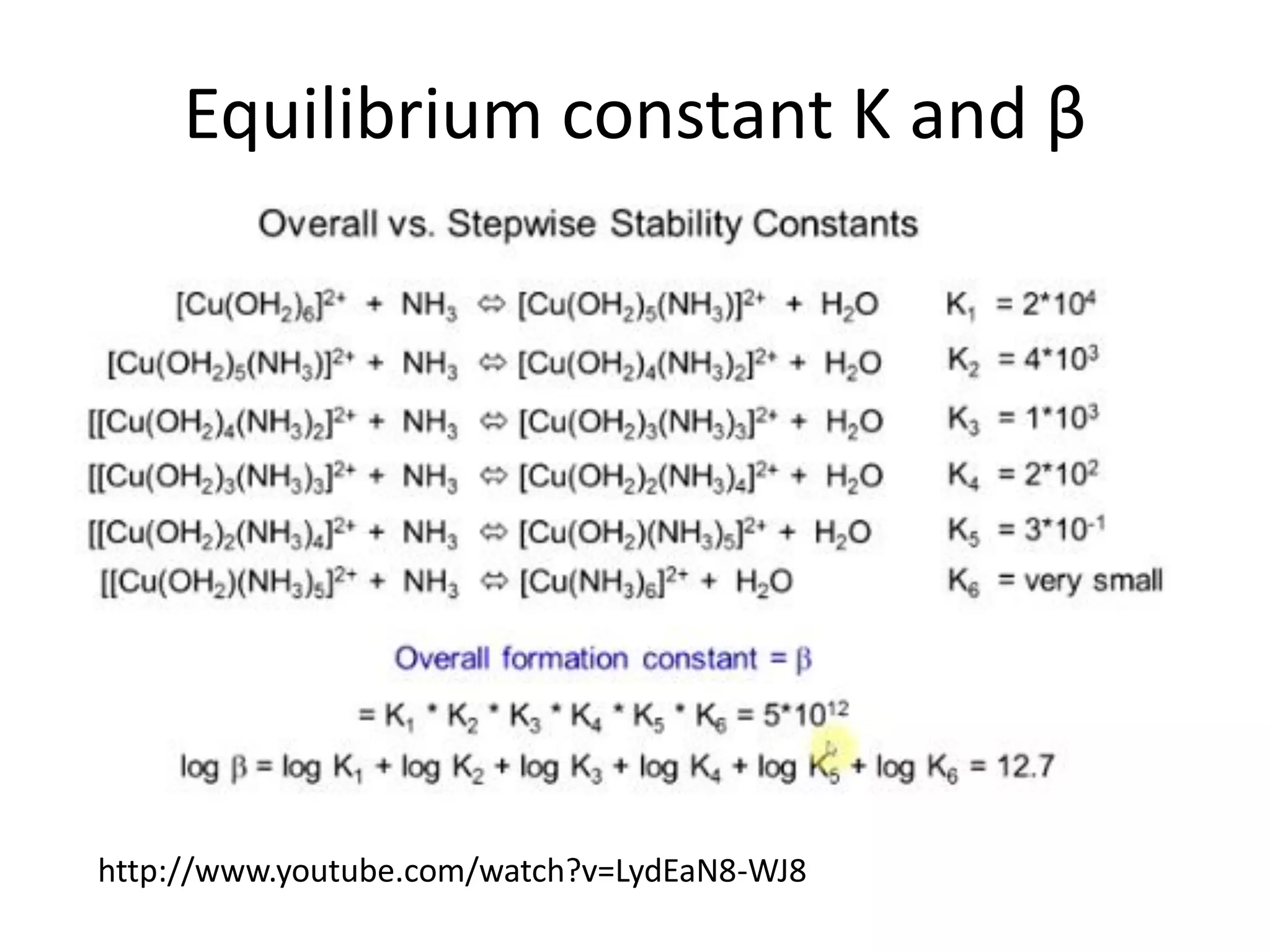
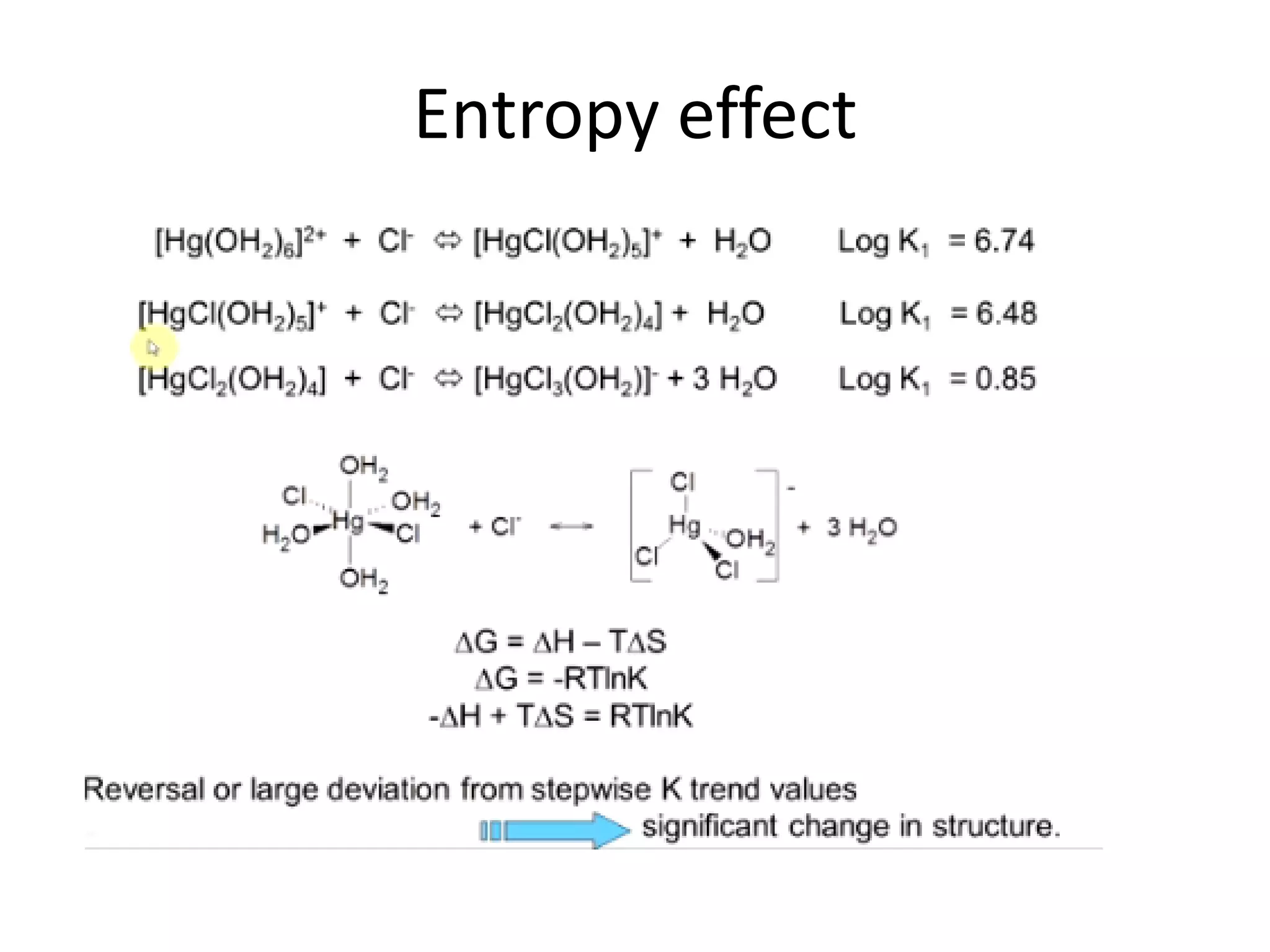

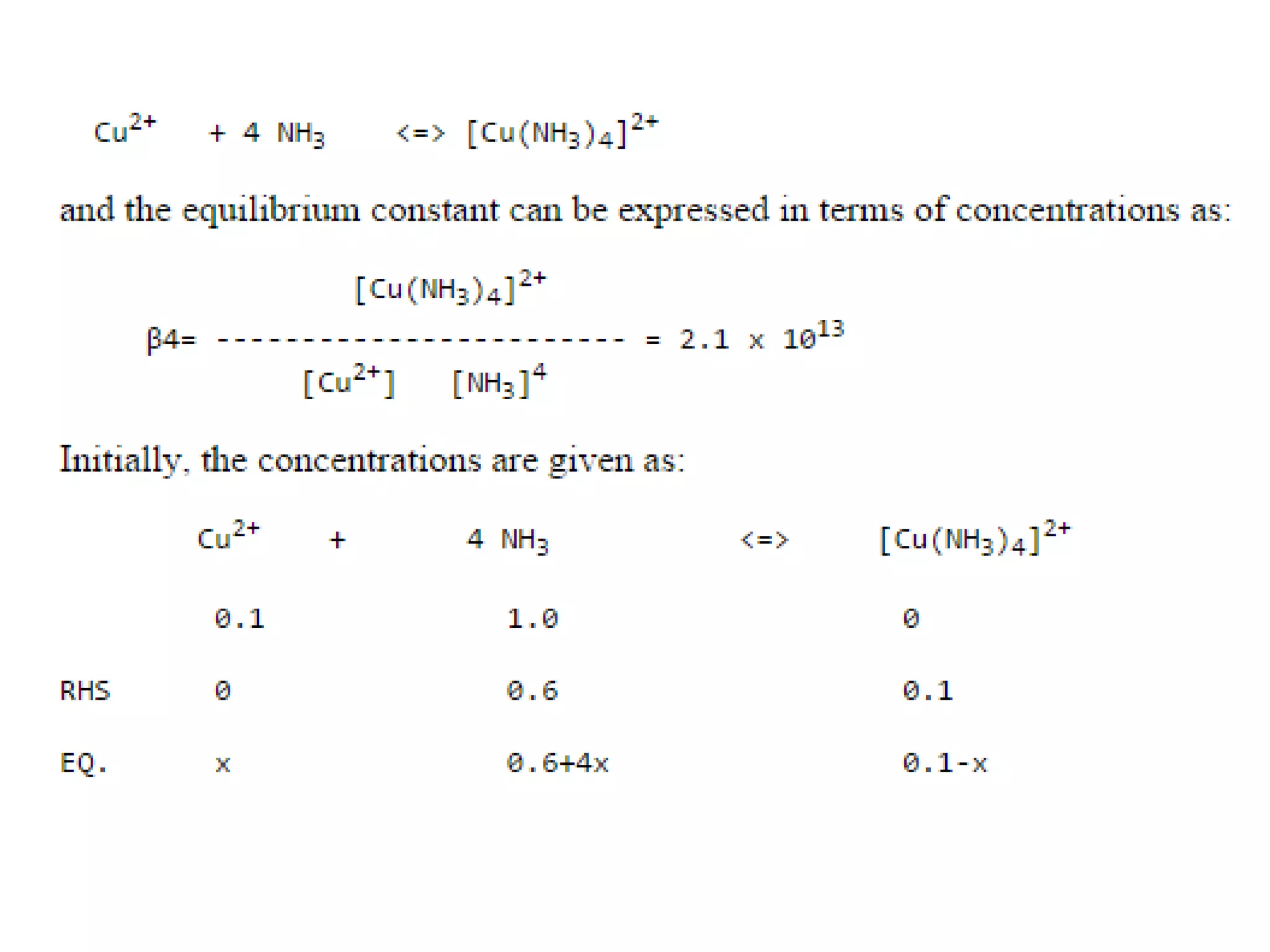
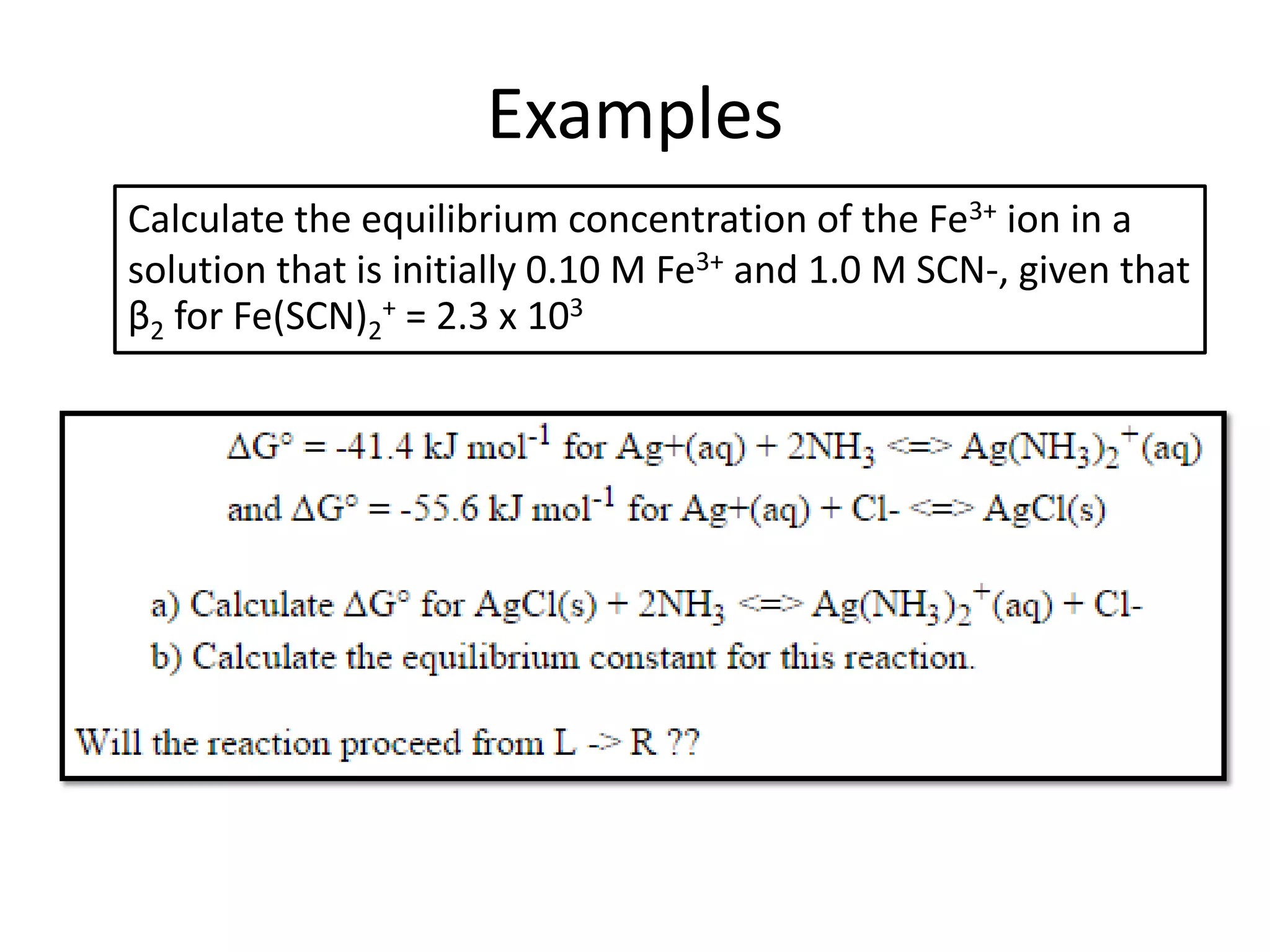
![Example 1
Assume that in the reaction of Cu2+ with ammonia,
the only complex ion to form is the tetra-ammine
species, [Cu(NH3)4]2+.
Given a solution where the initial [Cu2+] is 0.10M,
and the initial [NH3] is 1.0M and that β4 = 2.1 x 1013,
calculate the equilibrium concentration of the
Cu2+ ion.
http://wwwchem.uwimona.edu.jm/courses/IC10K1.html](https://image.slidesharecdn.com/properties-of-coordination-complexes-2014-160213161422/75/Properties-of-coordination-complexes-Complete-75-2048.jpg)