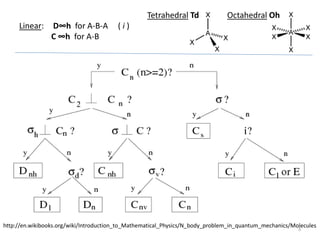
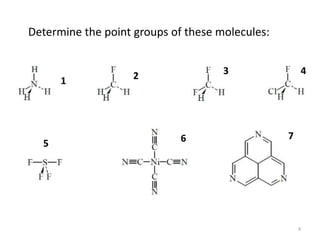
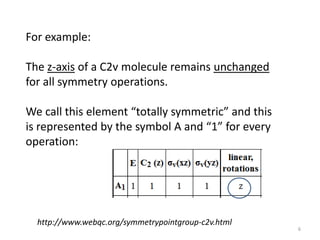
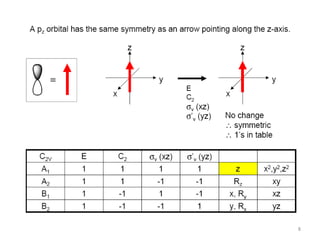
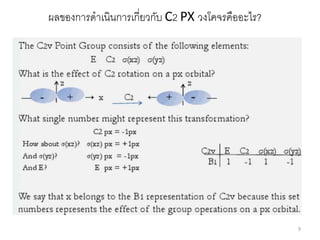
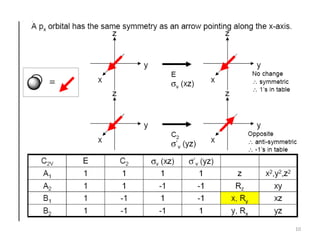
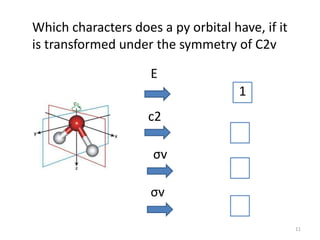
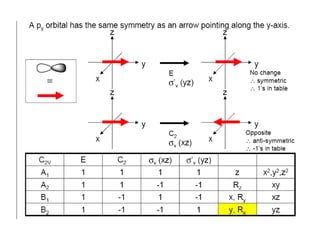
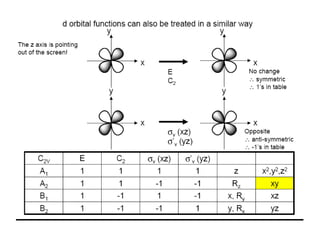
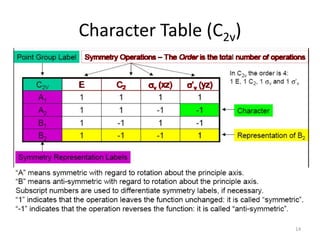
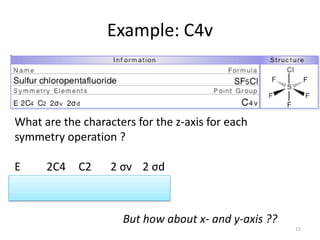
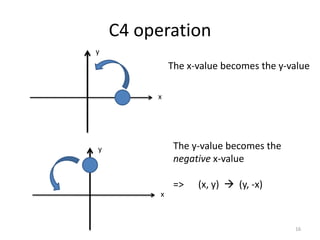
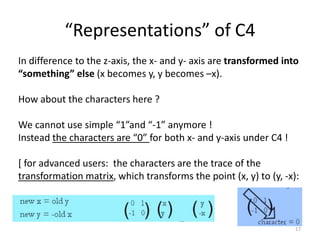
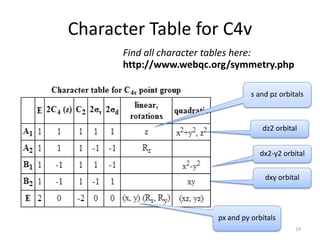
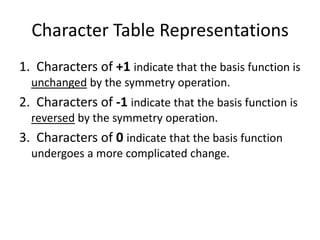
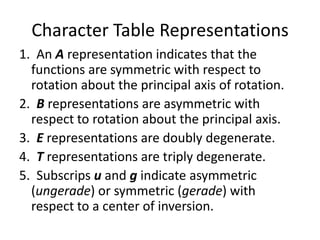
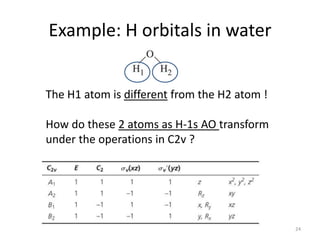
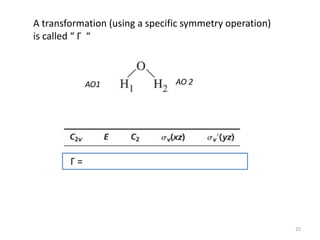
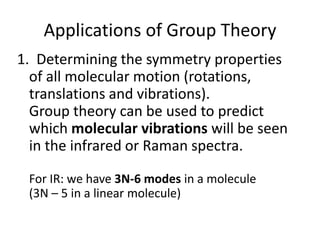
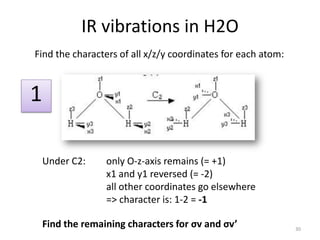
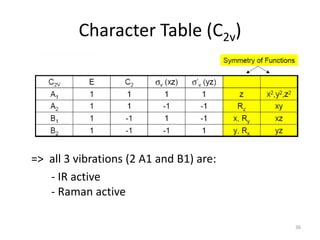
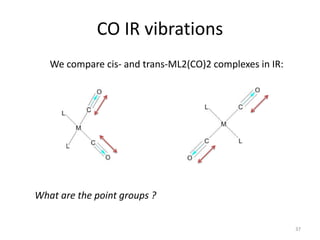
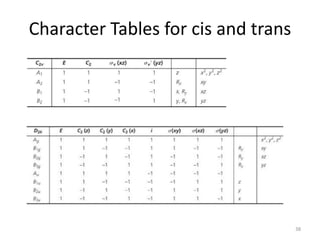
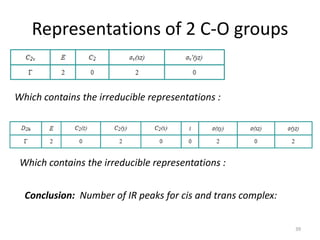
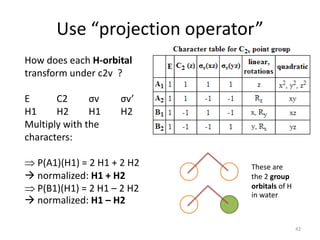
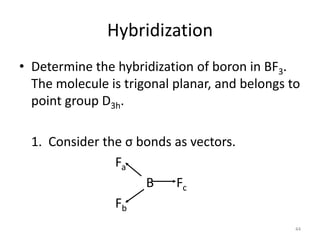
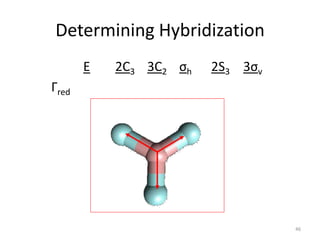
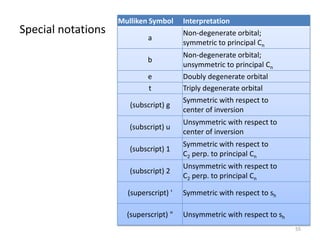
The document discusses character tables and symmetry operations in molecules. It provides examples of determining the point groups and irreducible representations of molecules like water. Character tables are used to predict molecular vibrations that will be active in infrared and Raman spectra. The document also discusses how group theory can be applied to determine hybridization of orbitals and molecular orbitals. Key applications of group theory covered are predicting vibrational modes, hybrid orbitals, and molecular orbitals of different symmetry.






![“Reduction” Formula – if we cannot see by just testing:
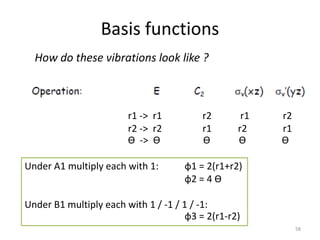
# A1 = (1/4) * [ (2 * 1 *1) + (0) + (2 * 1 *1) + (0) ] = 1
=> Γ contains 1x A1
# B1 = (1/4) * [ (2 *1 *1) + (0) + (2 *1 *1) ] = 1
=> Γ contains 1x B1
27
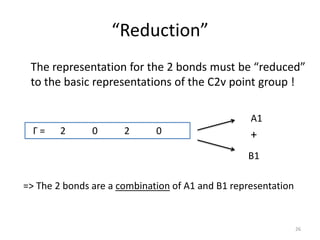
Г = 2 0 2 0
h = 4](https://image.slidesharecdn.com/character-tables-2013-160213160556/85/Character-Tables-in-Chemistry-27-320.jpg)

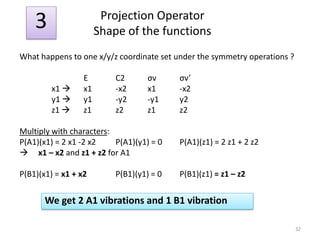
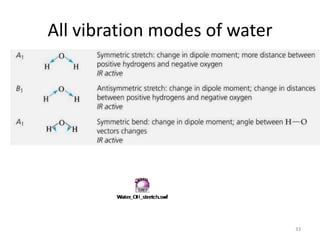
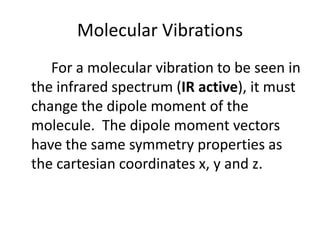
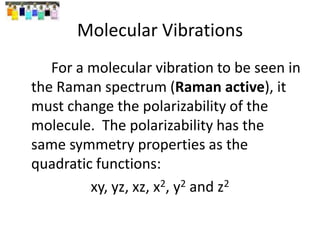



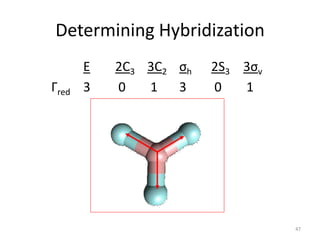
![“Reduction” of Гred
# A1’ = 1/12 *
[ ( 3 * 1 * 1) + ( 0) + (1 * 1 * 3) +
( 3 * 1 * 1) + (0) + ( 1 *1 *3)] = 1
Try the same method for A2’ and E’ !
48](https://image.slidesharecdn.com/character-tables-2013-160213160556/85/Character-Tables-in-Chemistry-48-320.jpg)
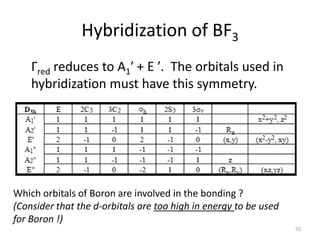
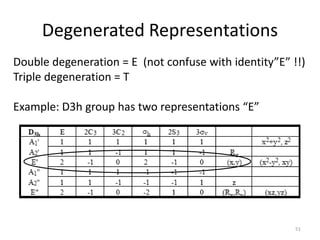
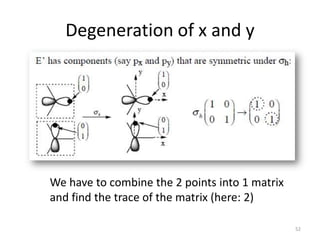
![“Reduction”
This is a reducible representation, so use character table to reduce it.
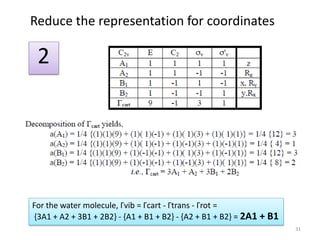
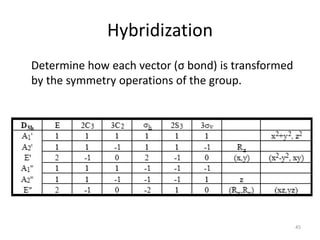
D3h E 2C3 3C2 σh 2S3 3σv
Г 3 0 1 3 0 1
#A1’ = (1/12) [ 3 + 0 + 3 + 3 + 0 + 3] = 1
#A2’ = (1/12) [ 3 + 0 - 3 + 3 + 0 - 3] = 0
…
#E’ = (1/12) [ 6 + 0 + 0 + 6 + 0 + 0] = 1
Г = A1’ + E’
Which orbitals belong to these symmetry species?
A1’ = s-orbital
E’ = 2 p-orbitals
Therefore, it is an sp2 hybrid orbital 49](https://image.slidesharecdn.com/character-tables-2013-160213160556/85/Character-Tables-in-Chemistry-49-320.jpg)
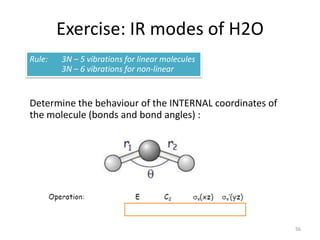
![Reducing Г
57
# A1 = ¼ [ (3) + (1) + (3) + (1) ] = 2
# B1 = ¼ [ (3) + (-1) + (3) + (-1) ] = 1](https://image.slidesharecdn.com/character-tables-2013-160213160556/85/Character-Tables-in-Chemistry-57-320.jpg)