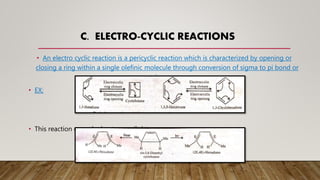
This document discusses applications of frontier molecular orbital theory, including cycloaddition reactions, sigma-tropic reactions, and electrocyclic reactions. Cycloaddition reactions like the Diels-Alder reaction can be predicted by Woodward-Hoffmann rules and frontier molecular orbital theory. Sigma-tropic reactions involve breaking a sigma bond and forming a new sigma bond with pi electron rearrangement. Electrocyclic reactions involve opening or closing a ring through conversion of sigma to pi bonds or vice versa. Frontier molecular orbital theory can be used to understand the orbital interactions in these pericyclic reactions. However, the theory has limitations and may not accurately predict reactivity in all cases.
![A. CYCLO-ADDITION REACTIONS
• A cycloaddition is a reaction that simultaneously forms at least two new bonds, and in
doing so, converts two or more open-chain molecules into rings.
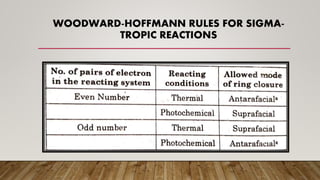
• These reactions can be predicted by the Woodward–Hoffmann rules and thus are closely
approximated by FMO Theory.
• Ex: The famous Diels-Alder reaction ,
where a diene of 4pi electrons reacts
with a dienophile of 2 pi electrons is
[4+2] cycloaddition.
The dimerization of ethylene is
a combination of two olefin
units and have a [2+2]
cycloaddition.](https://image.slidesharecdn.com/7982-200714112743/85/Frontier-Molecular-Orbital-Theory-3-320.jpg)
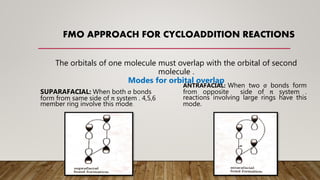
![[4,2] CYCLOADDITION
REACTIONS
Frontier orbital analysis of these reactions
show that overlap of in-phase orbital to form
the two new sigma bonds require suparafacial
orbital overlap .
There are bonding interaction at the termini.
The [Π2a + Π2S] addition is thermally or
symmetry allowed.
Ex:](https://image.slidesharecdn.com/7982-200714112743/85/Frontier-Molecular-Orbital-Theory-5-320.jpg)
![[2,2] CYCLOADDITION
REACTIONS
These reactions does not occur under thermal
conditions but take place under photochemical
conditions.
The reason isUnder thermal conditions , suprafacial overlap is
not allowed . Antraafacial is allowed ,but not
possible due to small size ring.
Under photochemical conditions , suparafacial
bond formation is allowed because excited state
HOMO have symmetry opposite to ground state
HOMO.](https://image.slidesharecdn.com/7982-200714112743/85/Frontier-Molecular-Orbital-Theory-6-320.jpg)

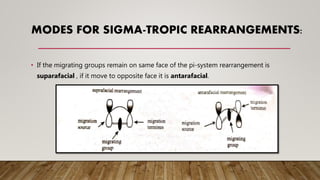
![TYPES OF REARRANGEMENTS
A [ 2,3 ]
sigma-tropic
rearrangement:
A [ 1,5 ] sigma-
tropic
rearrangement:
A [ 1,3 ] sigma-
tropic
rearrangement:
A [ 3,3 ]
sigma-tropic
rearrangement:
• h](https://image.slidesharecdn.com/7982-200714112743/85/Frontier-Molecular-Orbital-Theory-8-320.jpg)