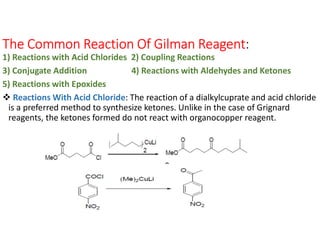
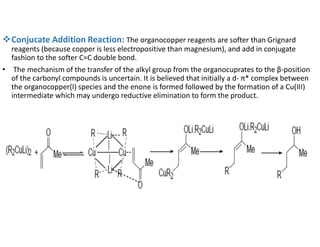
Gilman reagent, also known as organocopper reagents, are prepared by reacting organomagnesium, organolithium, or organozinc reagents with copper(I) salts. Gilman reagents react with a variety of electrophiles including acid chlorides, aldehydes, ketones, epoxides, and alkyl halides. Some common reactions of Gilman reagents are: 1) reactions with acid chlorides to form ketones, 2) coupling reactions between two different alkyl halides to form C-C bonds, and 3) conjugate addition reactions of the organocopper reagent to unsaturated carbonyl compounds like enones. Gilman reagents offer advantages over Grignard reagents for