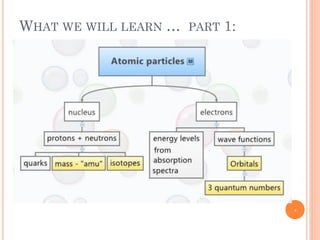
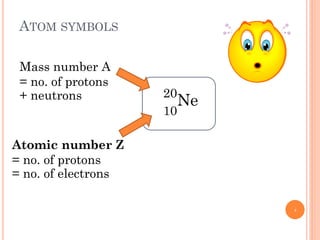
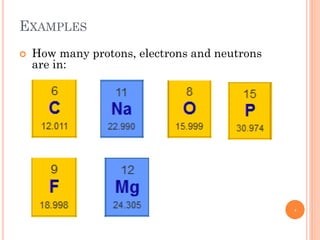
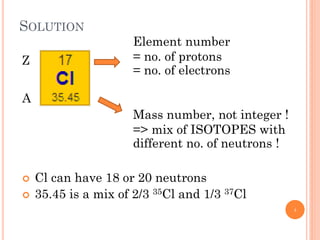
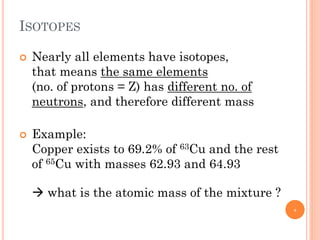
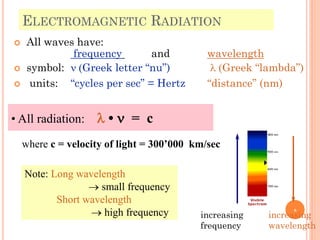
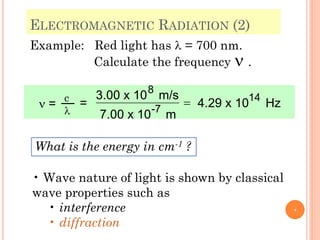
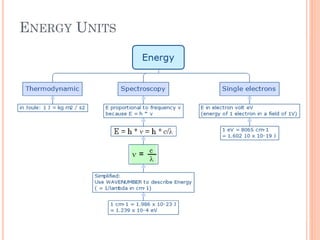
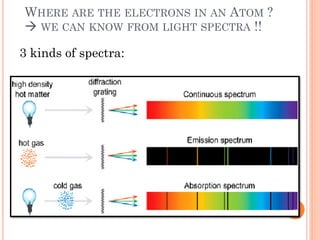
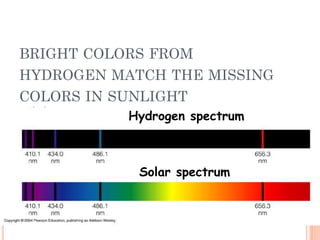
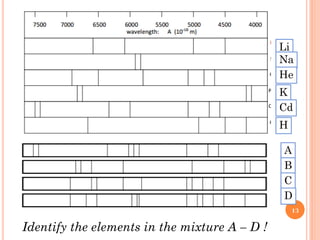
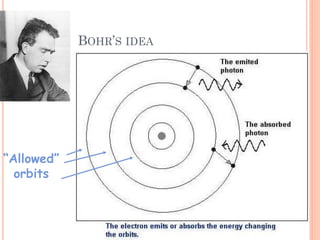
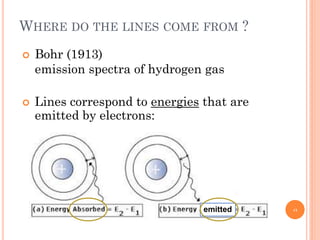
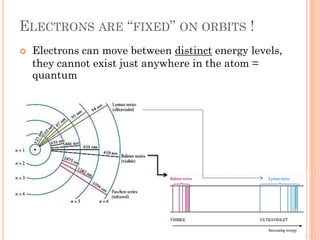
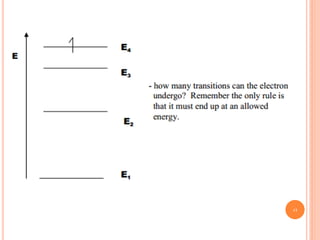
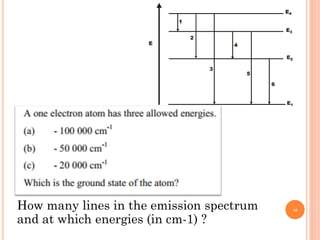
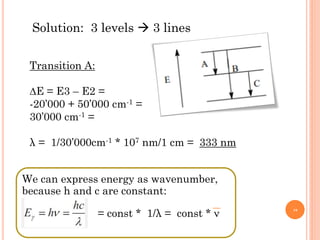
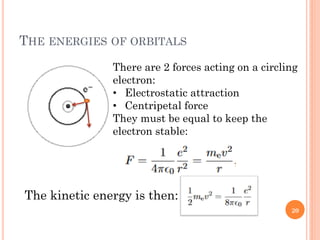
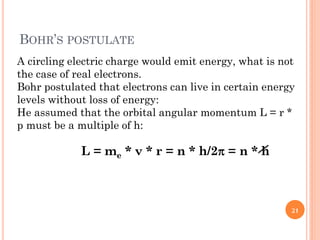
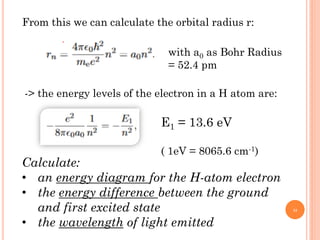
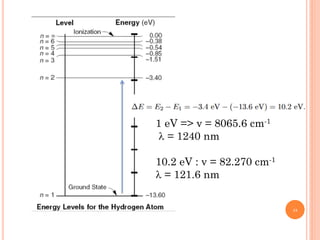
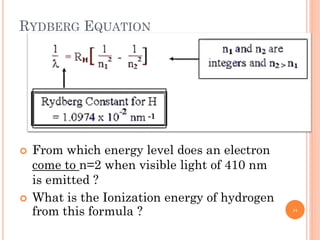
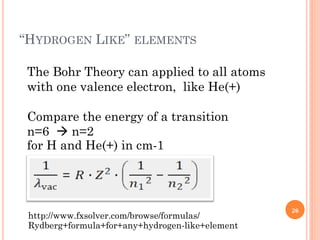
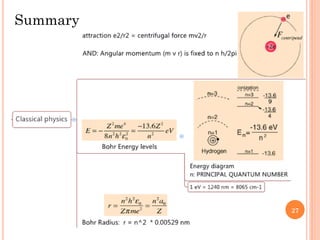
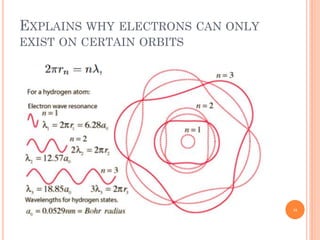
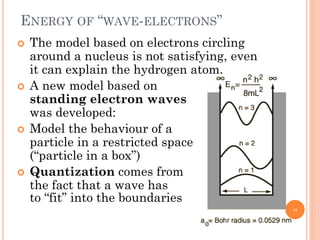
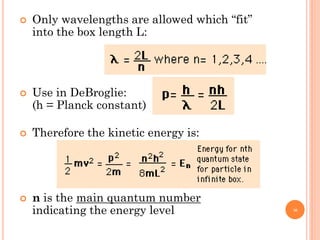
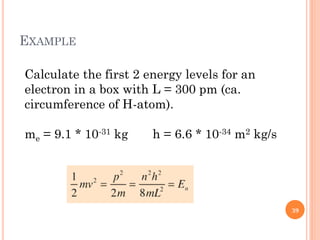
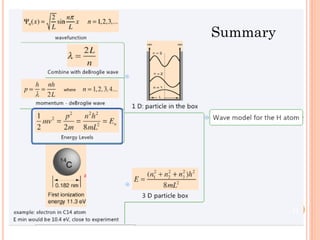
1) Electrons in atoms can only exist at certain discrete energy levels called quantum states. This is because electrons behave like waves and their wavelengths must fit within the boundaries of the atom.
2) Niels Bohr used this idea to explain the emission spectrum of hydrogen, showing electrons jumping between allowed orbits.
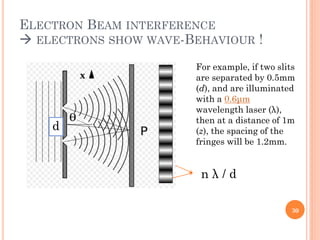
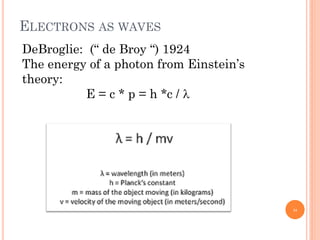
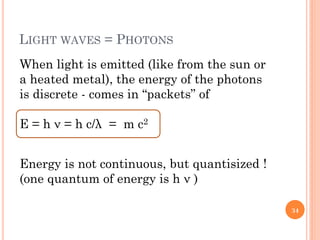
3) Later, the de Broglie hypothesis established that all particles like electrons exhibit both wave and particle properties. Treating electrons as waves led to the modern quantum mechanical model of atomic structure.