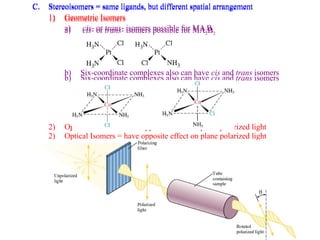
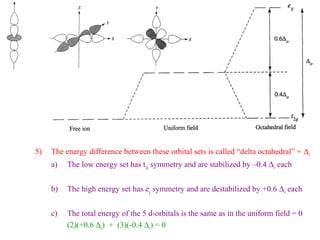
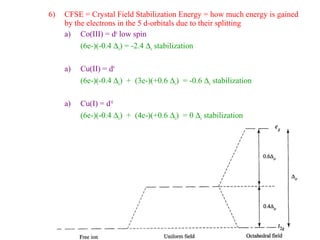
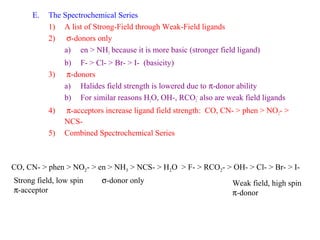
This document discusses the nomenclature and isomers of coordination complexes. It begins by defining ligands and describing systems for naming complexes based on the central metal ion, ligands, and ligand coordination. It then describes three types of isomers that can occur in complexes: structural isomers which have different ligands, linkage isomers which have the same ligands bonded differently, and stereoisomers which have the same ligands in different spatial arrangements. The document goes on to describe crystal field theory and ligand field theory, which explain the splitting of d-orbital energies in an octahedral ligand field based on ligand type. Strong-field ligands create a large energy gap between t2g and eg* orbitals, while weak-field ligands create
![B. Naming and Writing Formulas of Coordination Compounds
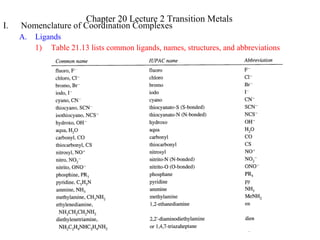
1) The cation comes first, then the anion(s)
a) diamminesilver(I) chloride [Ag(NH3)2]Cl
b) potassium hexacyanoferrate(III) K3[Fe(CN)6]
2) Inner Sphere Complex Ion is enclosed in brackets
a) Ligands are named before the metal
b) Metal is written first in the formula
c) Metal oxidation state in Roman Numerals in parenthesis after the metal ion
d) A space only between cation and anion
e) No capitalization is needed
i. tetraamminecopper(II) sulfate [Cu(NH3)4]SO4
ii. hexaamminecobalt(III) chloride [Co(NH3)6]Cl3
3) Prefixes denote the number of each ligand type. Special prefixes and parentheses
are used if the ligand already contains a prefix.
2 di bis 6 hexa hexakis
3 tri tris 7 hepta heptakis
4 tetra tetrakis 8 octa octakis
5 penta pentakis 9 nona nonakis
10 deca decakis](https://image.slidesharecdn.com/transitionmetals-130904032125-/85/Transition-metals-2-320.jpg)
![a) dichlorobis(ethylenediamine)cobalt(III) fluoride [Co(en)2Cl2]F
b) tris(bipyridine)iron(II) chloride [Fe(bipy)3]Cl2
4) Ligands are named in alphabetical order not counting prefixes.
a) tetraamminedichlorocobalt(III) [Co(NH3)4Cl2]+
b) amminebromochloromethylamineplatinum(II) [Pt(NH3)BrCl(CH3NH2)]
5) Ligand name alterations:
a) Anionic ligands are given an -o suffix: chloro, flouro, oxo, sulfato
b) Neutral ligands keep their name: methylamine, bipyridine
c) Water becomes aqua
d) NH3 becomes ammine to keep separate from alkylamines
6) How to handle anionic complexes
a) Add –ate to the metal name if the complex ion has an overall (-) charge
b) Negatively charged complexes of certain metals use their Latin names:
Fe = ferrate Ag = argenate Sb = stibate
Pb = plumbate Sn = stannate Au = aurate
c) [PtCl4]2-
= tetrachloroplatinate(II)](https://image.slidesharecdn.com/transitionmetals-130904032125-/85/Transition-metals-3-320.jpg)

![B. Structural Isomers = different ligands in coordination sphere
1) Coordination Isomers = ratio of ligand:metal same, but ligands are attached to
metal ions in different numbers
a) [Pt(NH3)2Cl2]
b) [Pt(NH3)3Cl][Pt(NH3)Cl3]
c) [Pt(NH3)4][PtCl4]
2) Linkage Isomers = depends on which atom of the ligand is attached to metal
a) SCN-
Linkage isomers
i. Pb2+
—SCN = thiocyanate complex
ii. Fe3+
—NCS = isothiocyanate complex
b) NO2
-
Linkage isomers
i. M—ONO = nitrito complex
ii. M—NO2 = nitro complex](https://image.slidesharecdn.com/transitionmetals-130904032125-/85/Transition-metals-5-320.jpg)