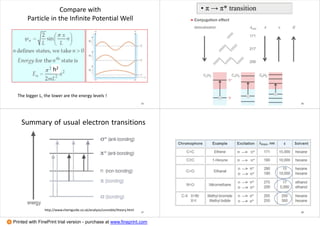
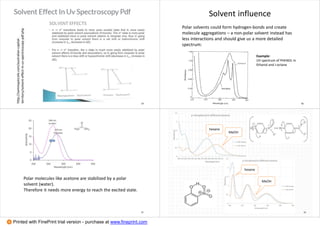
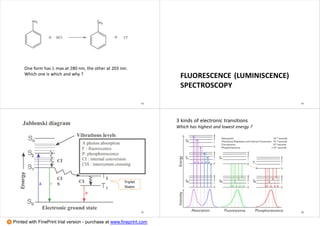
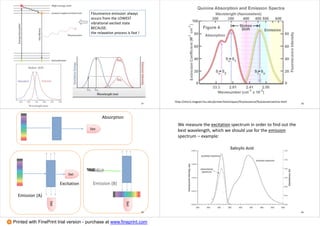
1. The document discusses principles of spectroscopy, including absorption spectroscopy which measures light absorbed by a sample (e.g. UV-Vis, IR, NMR) and emission spectroscopy which measures light emitted from an excited sample (e.g. fluorescence, Raman spectroscopy).
2. It covers electronic transitions in molecules, character tables, determining symmetry of molecular orbitals, and which transitions are allowed. Singlet and triplet states are also discussed.
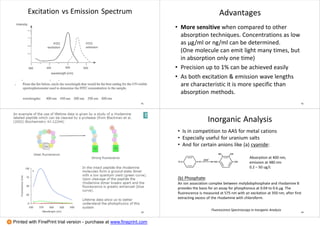
3. Advantages of fluorescence spectroscopy are highlighted, including high sensitivity down to the microgram level and specificity from characteristic excitation and emission wavelengths. Examples of applications to inorganic analysis of metals and anions are provided.



![State of the molecule:
b2 x b2 = A1
b2
b2
b1
b1 x b2 = A2Molecules with all
electrons paired are
always totally symmetric
Symmetry of the whole molecule:
21
Instead of saying:
One electron moved from b1 (HOMO) to b2 (LUMO)
[which is not allowed since b1 x b2 = a2]
We can also say:
The molecule changed its state from A1 (ground state)
to A2 (excited state)
or: A1 -> A2*
Electronic Transitions:
22
Example 2: Butadiene
23
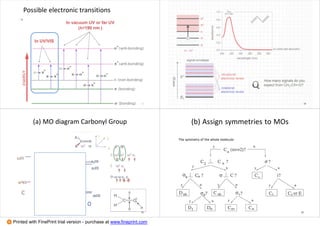
Which transitions are allowed ?
(1) Determine the symmetry of the molecule
(2) Look up the character table
(3) Determine the characters for the HOMO and the two
LUMOs
(4) Multiply the characters for HOMO with each LUMOs
(5) Look up if these characters contain x,y or z
component
24
Printed with FinePrint trial version - purchase at www.fineprint.com](https://image.slidesharecdn.com/uvspectroscopy2019part1compact-190728141502/85/Inorganic-spectroscopy-2019-part-1-compact-6-320.jpg)