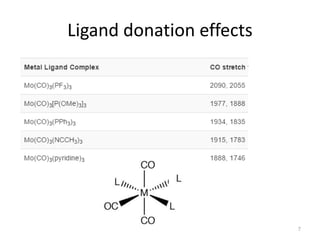
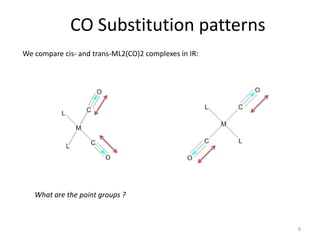
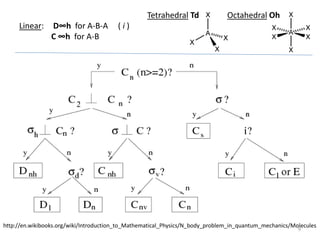
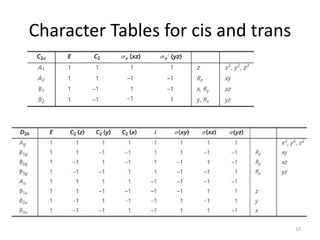
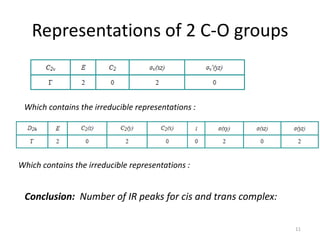
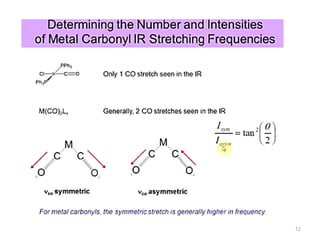
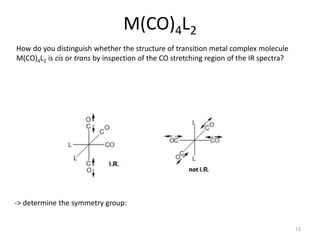
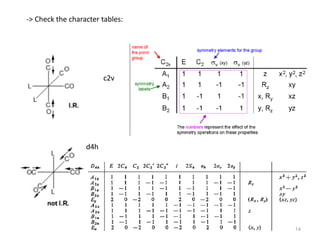
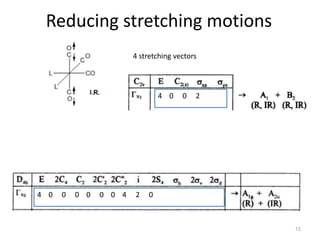
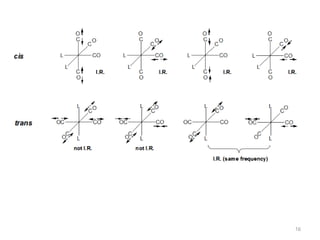
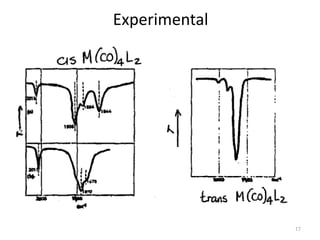
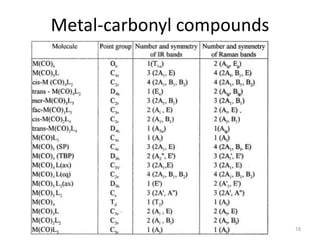
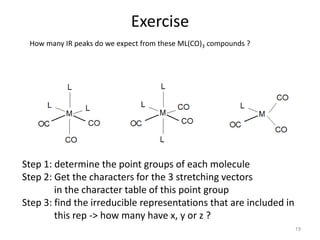
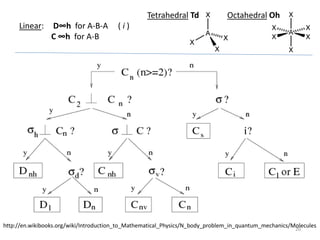
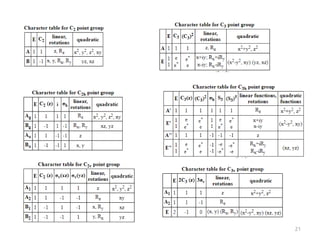
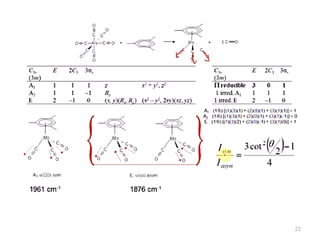
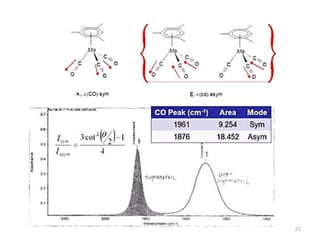
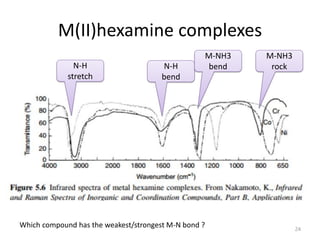
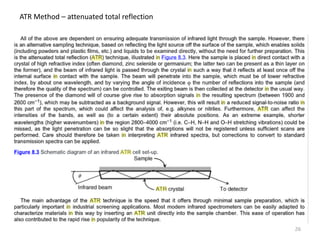
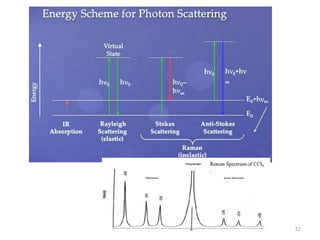
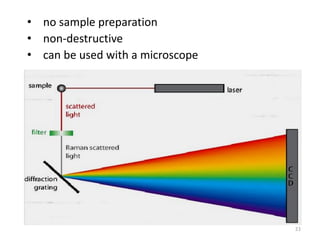
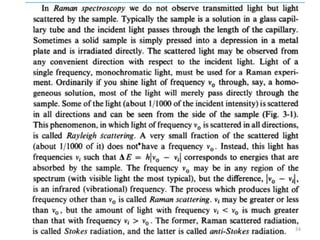
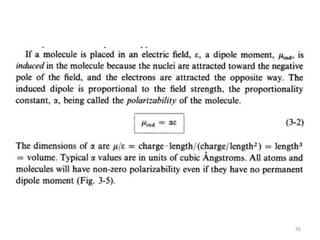
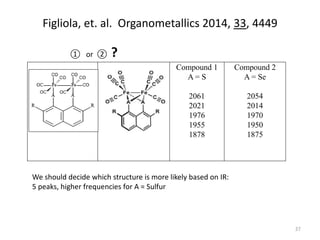
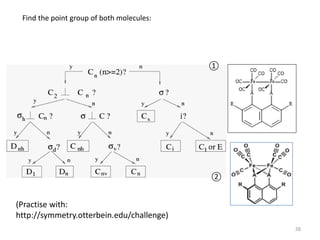
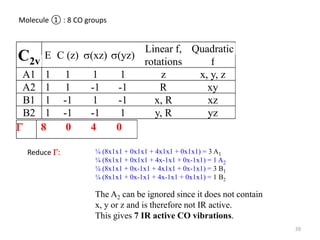
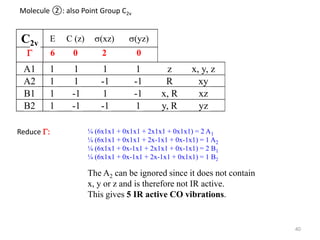
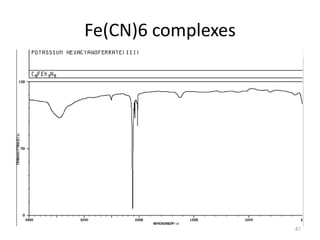
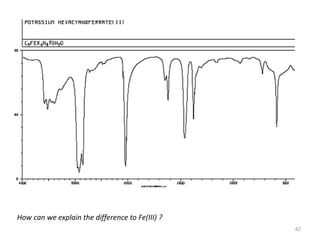
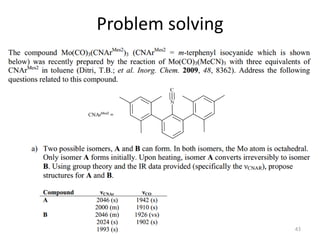
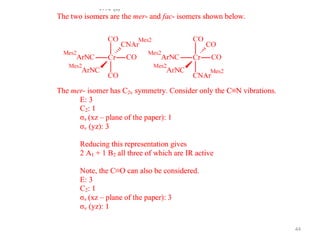
This document discusses spectroscopic methods for analyzing organometallic compounds, focusing on IR and Raman spectroscopy of metal carbonyl complexes. It explains that the C-O stretching wavenumbers in metal carbonyl complexes shift to lower values when there is more back-bonding from the metal to the carbonyl π* orbitals. The number of IR peaks expected for related complexes can be determined from the point group and character tables. It also briefly mentions using IR, Raman, UV-Vis, and NMR spectroscopy to study other types of inorganic complexes.
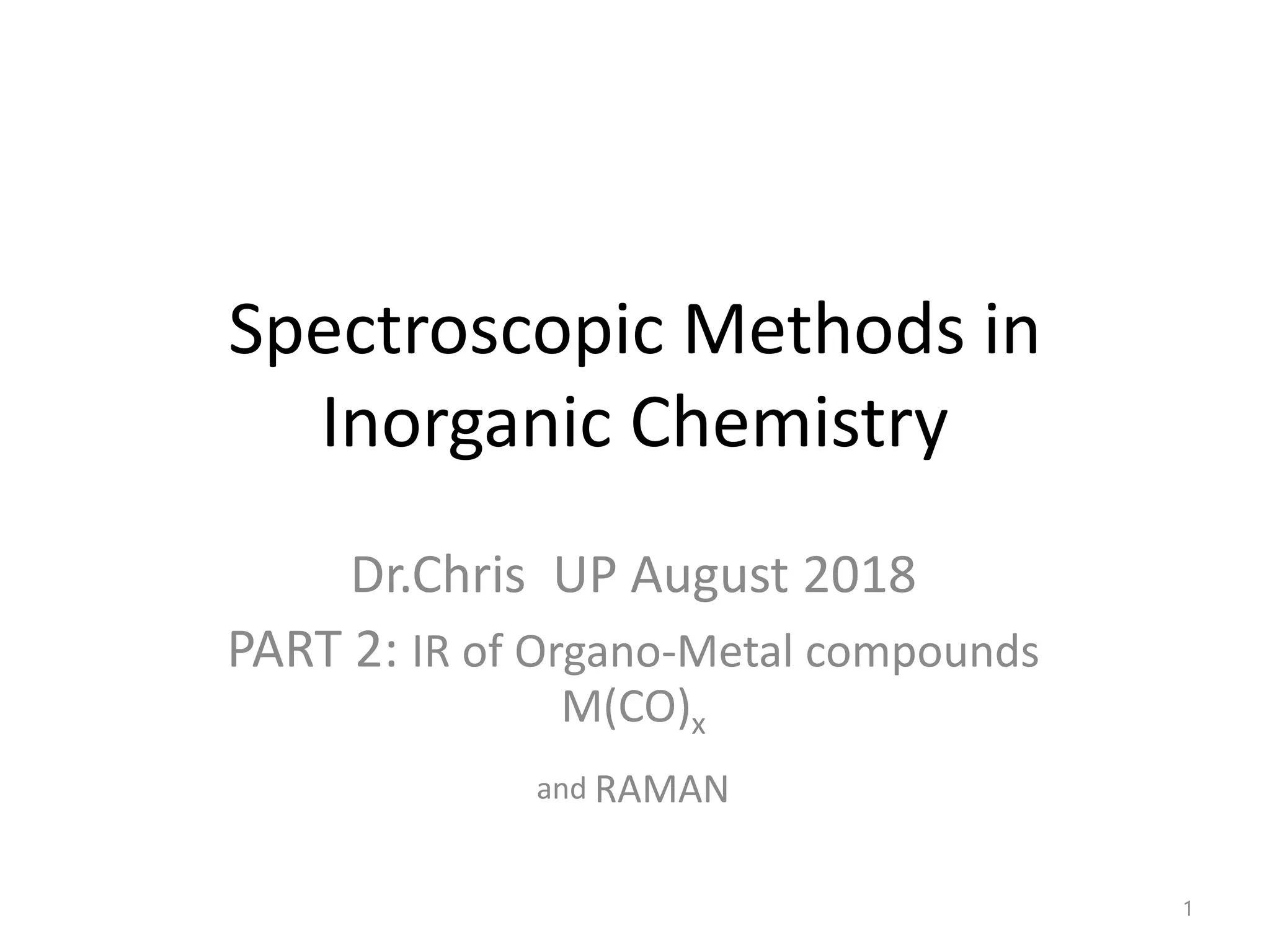
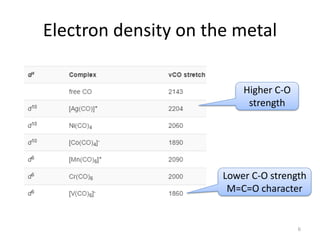
![The C–O stretching wavenumbers are shifted to lower values when
there are changes in the extent of back-bonding in the compound.
Removing positive charge from the metal causes the shift of
electrons from the metal to the CO π∗ orbitals causes the CO
wavenumber values to decrease.
The highest excess of negative charge on the metal occurs in the
*V(CO)6 +− complex and so more back-bonding occurs than in the
other complexes.
The next highest excess of electron density is in Cr(CO)6 , and then
[Mn(CO)6 ]+.
5](https://image.slidesharecdn.com/spectroscopicmethodsirpart2-180904065115/85/Spectroscopic-methods-IR-part-2-5-320.jpg)