This document discusses key concepts in atomic structure including:
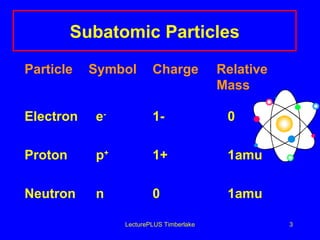
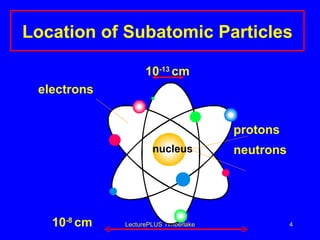
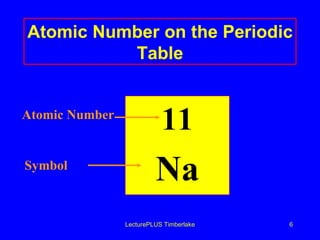
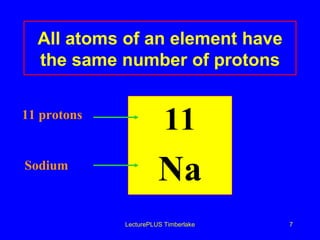
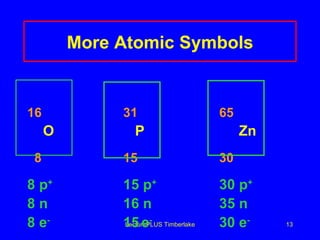
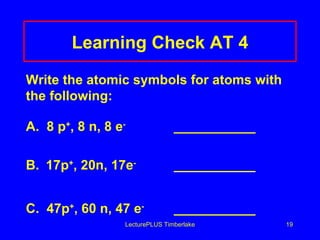
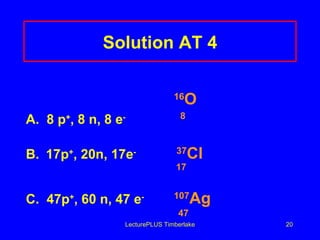
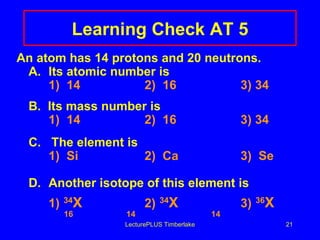
1) Atoms are made of subatomic particles including protons, neutrons, and electrons. The atomic number is the number of protons in an atom, which defines the element.
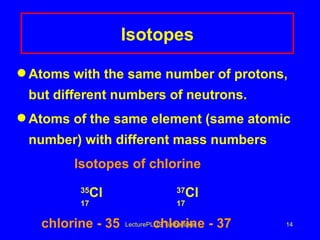
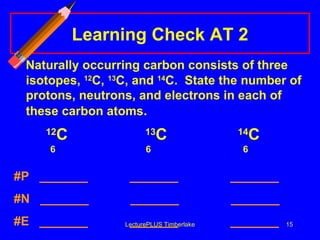
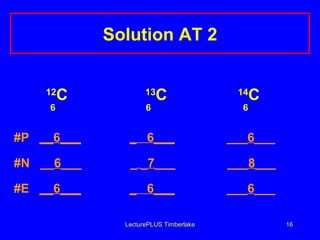
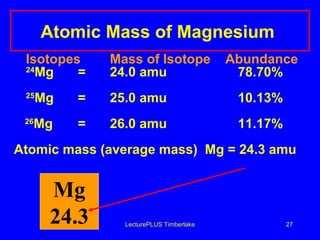
2) Isotopes are atoms of the same element that have different numbers of neutrons, giving them different mass numbers.
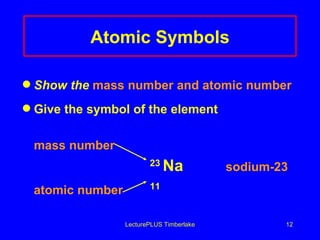
3) The mass number counts both protons and neutrons in an atom. Atomic symbols indicate the mass number and atomic number.