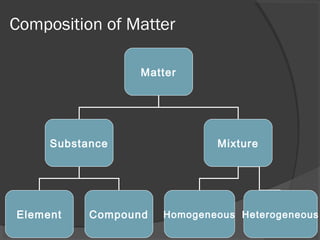
1. Matter is classified as either pure substances or mixtures. Pure substances are uniform and consist of elements or compounds, while mixtures contain two or more substances mixed together.
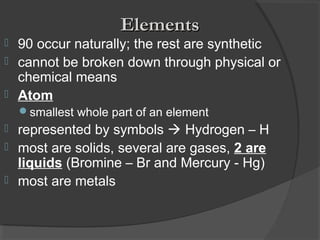
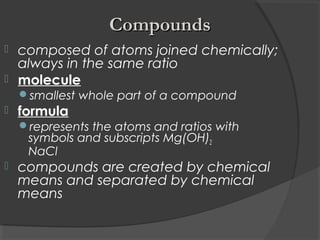
2. Elements are the simplest pure substances made of only one type of atom, while compounds are formed by chemical bonds between different atoms in specific ratios.
3. Mixtures can be either homogeneous, with substances mixed uniformly, or heterogeneous, with distinct parts. Homogeneous mixtures include solutions and colloids, while heterogeneous mixtures have visible parts that settle over time like suspensions.