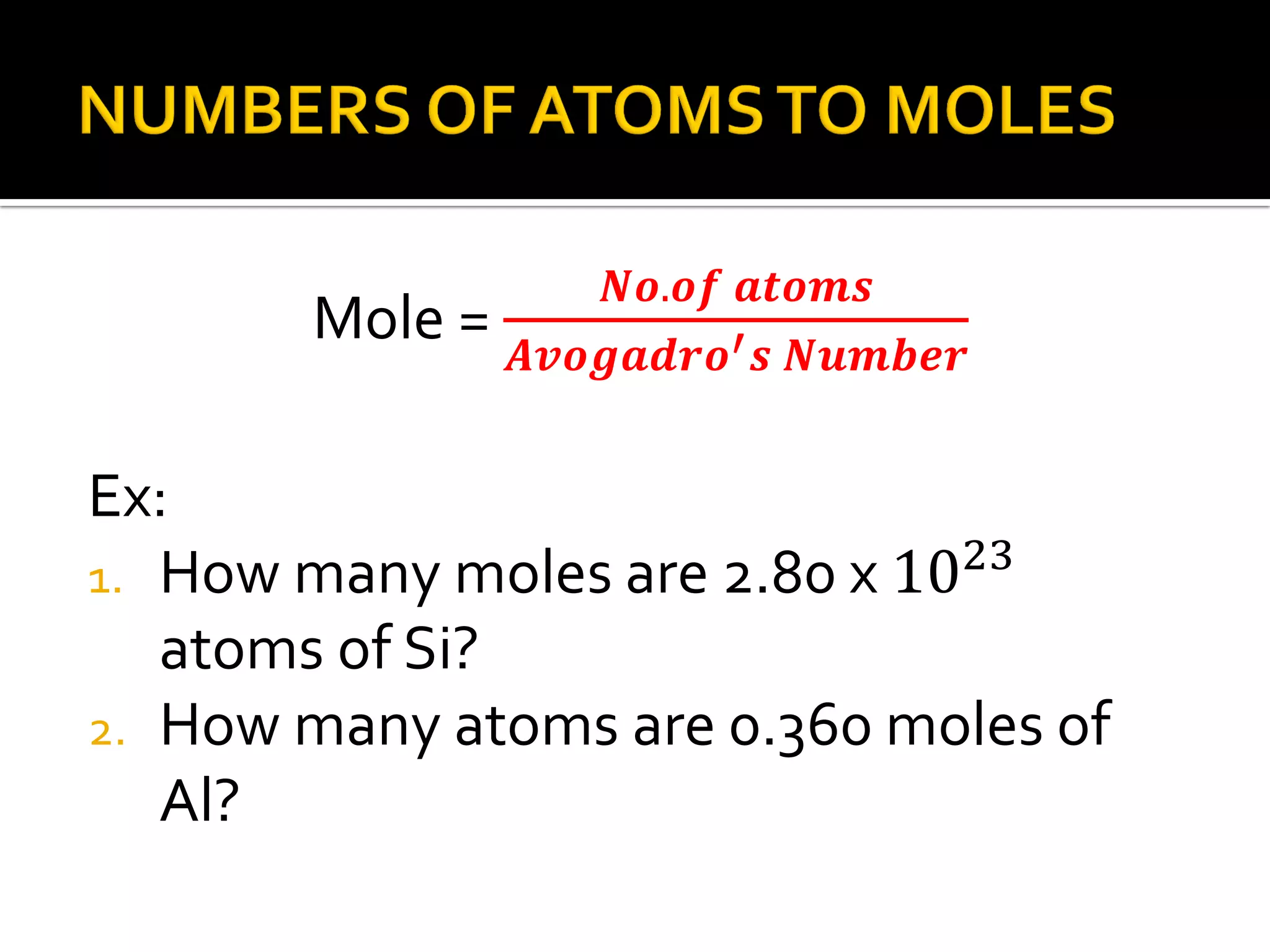
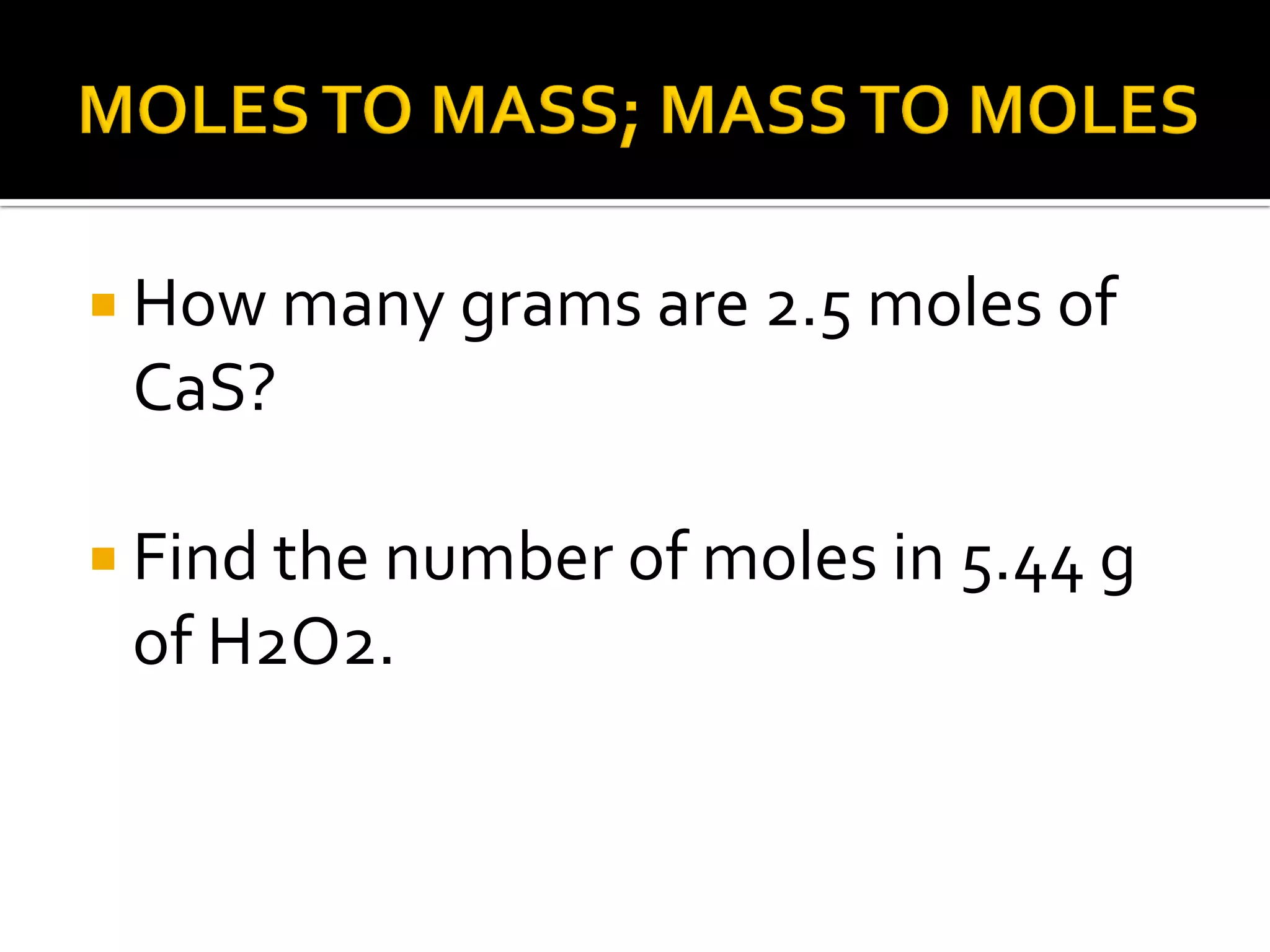The document defines key chemistry concepts related to moles, including:
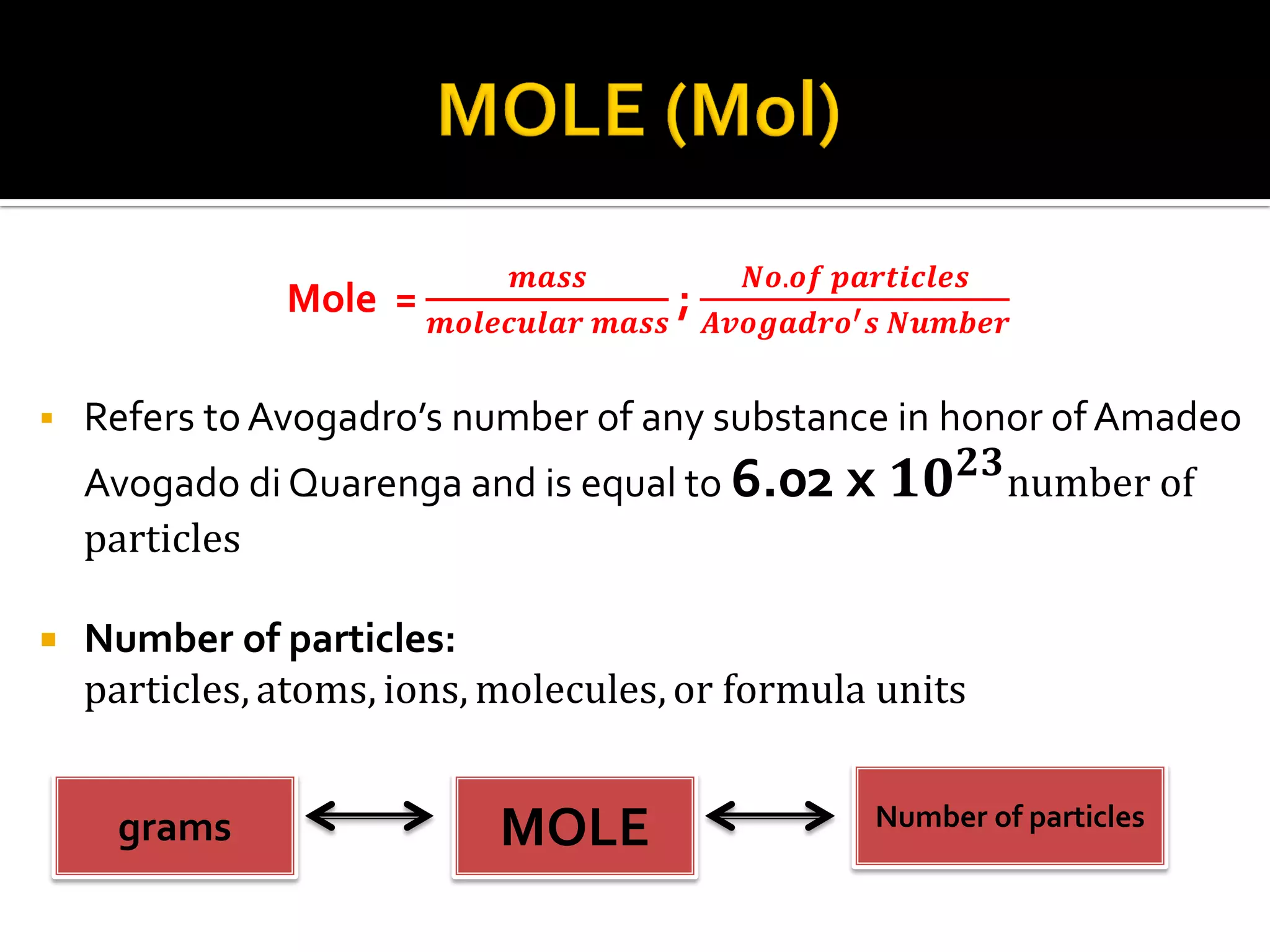
- A mole refers to Avogadro's number (6.02x10^23) of particles like atoms, molecules, ions or formula units.
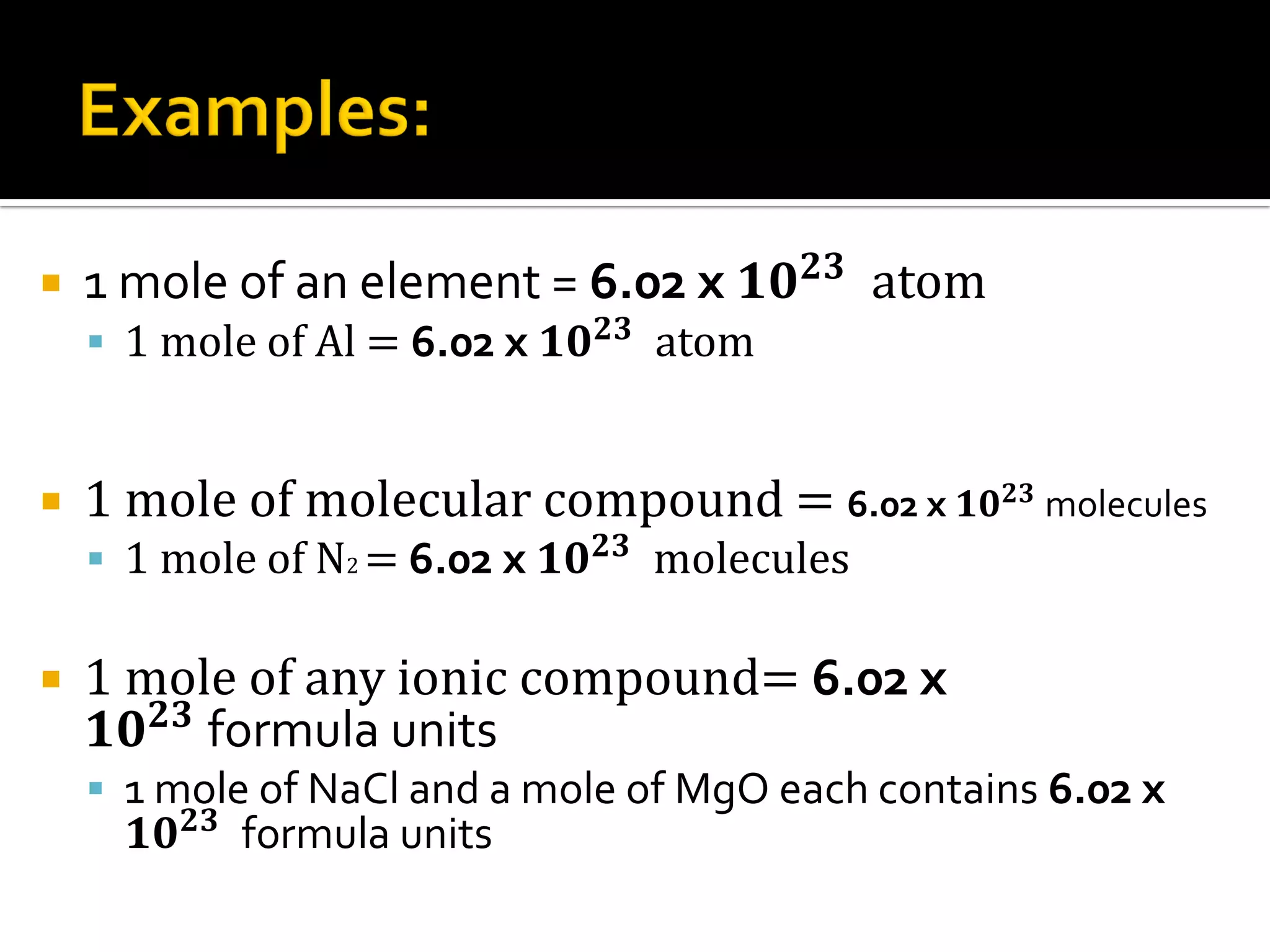
- 1 mole of an element contains 6.02x10^23 atoms, 1 mole of a molecular compound contains 6.02x10^23 molecules, and 1 mole of an ionic compound contains 6.02x10^23 formula units.
- Gram atomic mass refers to the mass of one mole of an element in grams, and gram formula mass refers to the sum of atomic weights that make up one mole of a compound.
- Chemical formulas represent the