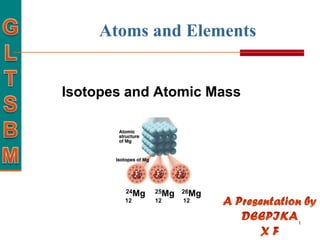
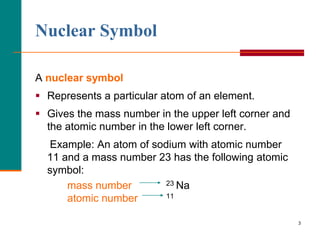
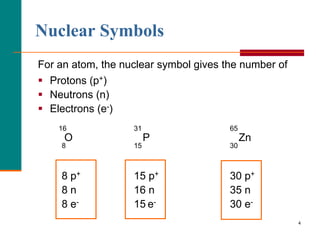
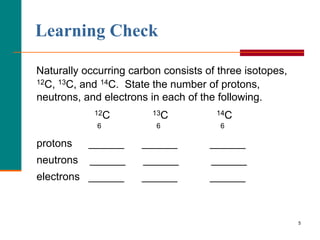
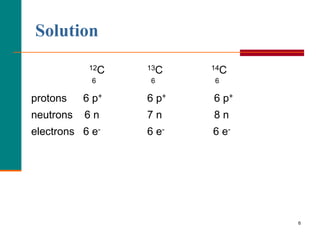
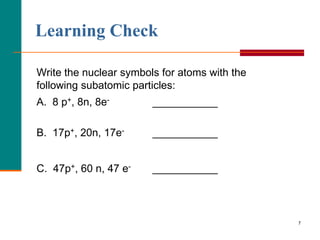
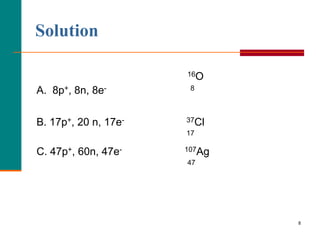
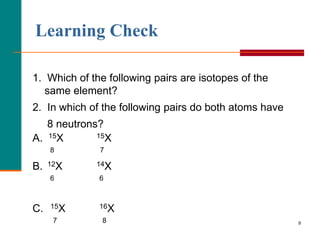
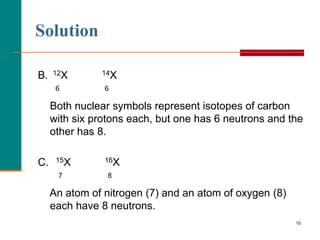
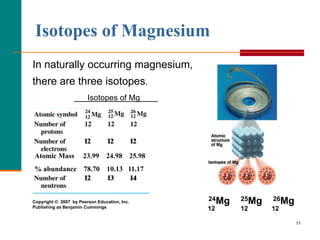
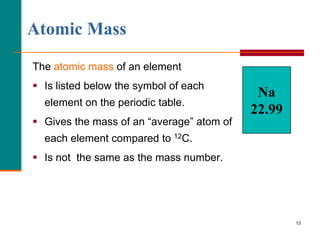
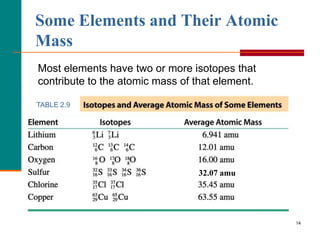
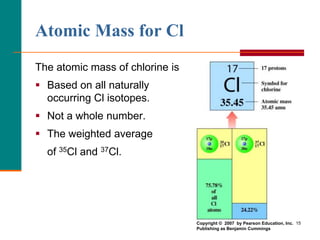
This document discusses isotopes and atomic mass. It defines isotopes as atoms of the same element that have different mass numbers due to varying numbers of neutrons. A nuclear symbol represents a particular atom and gives the mass number and atomic number. Examples are provided of writing nuclear symbols for atoms with given numbers of protons, neutrons, and electrons. The document also discusses that the atomic mass of an element is based on the naturally occurring isotopes and their abundances, and is provided for some common elements from the periodic table.