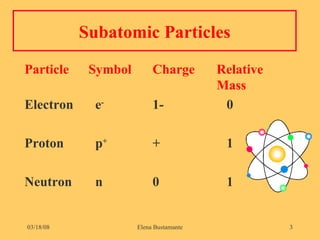
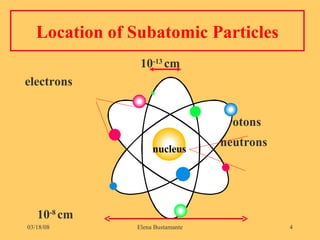
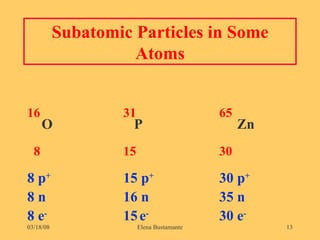
1) The document discusses the structure of atoms including subatomic particles like protons, neutrons, and electrons.
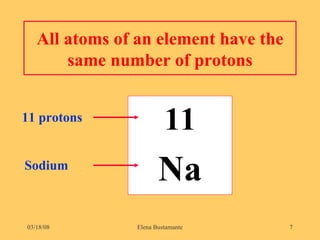
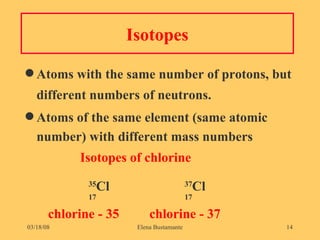
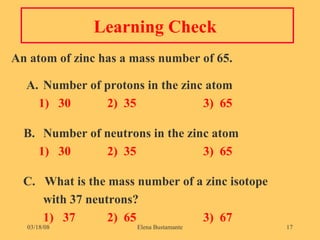
2) Atoms of the same element have the same number of protons but may have different numbers of neutrons, forming isotopes of that element.
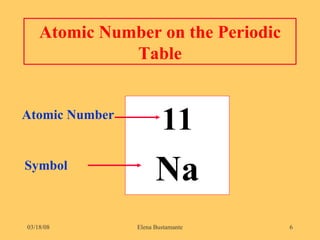
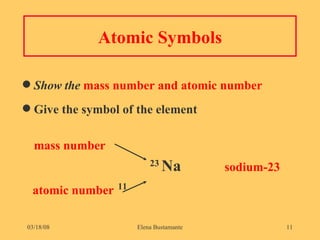
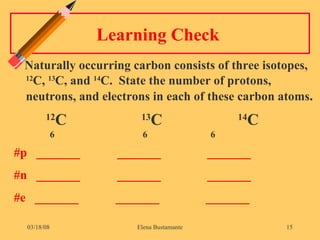
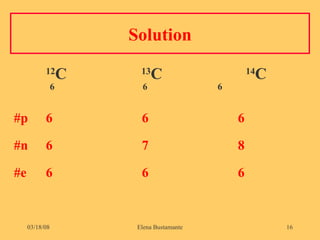
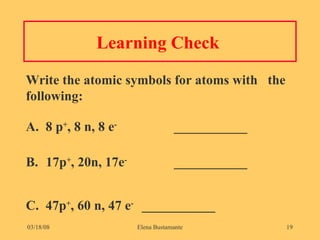
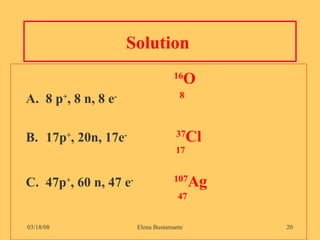
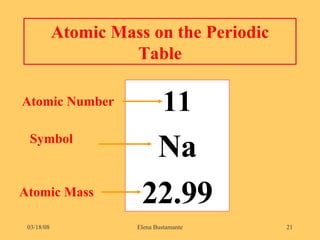
3) The atomic number represents the number of protons in an atom, while the mass number counts both protons and neutrons.