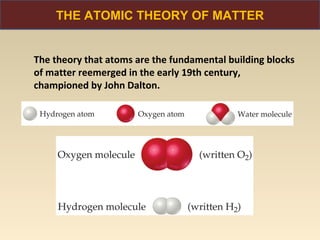
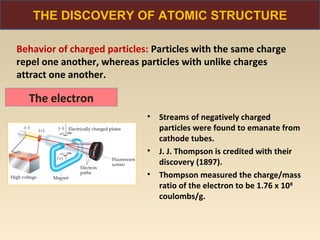
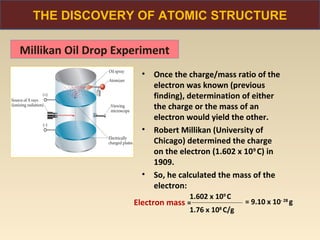
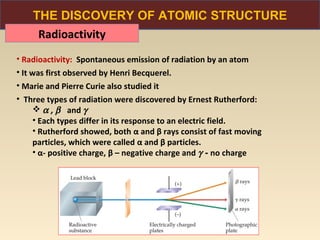
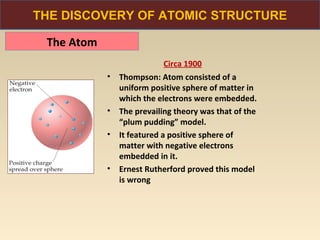
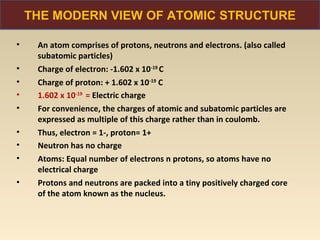
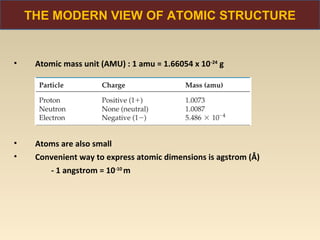
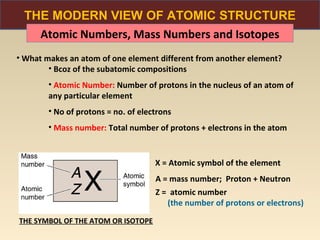
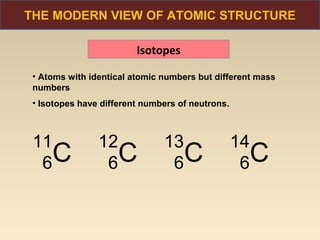
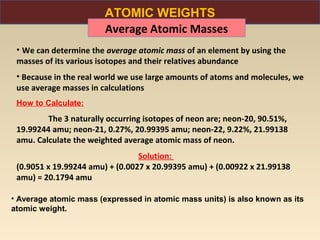
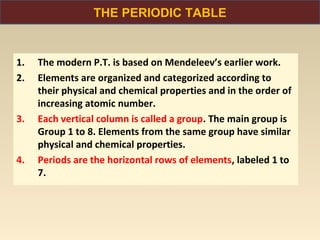
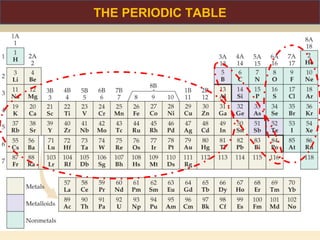
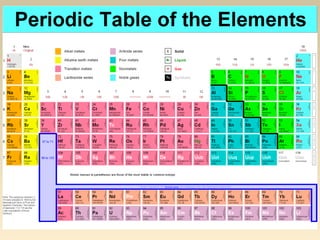
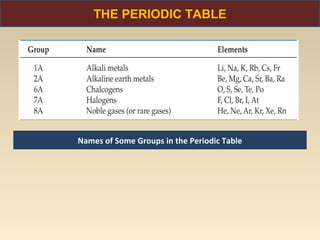
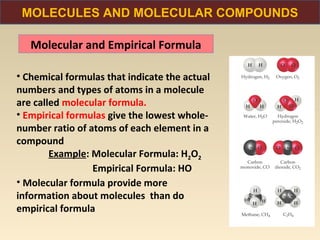
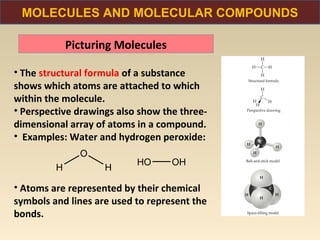
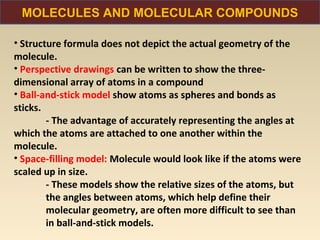
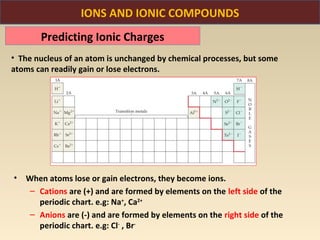
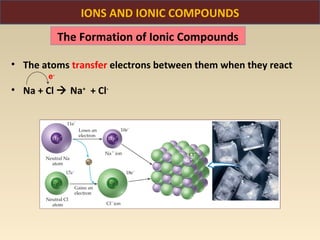
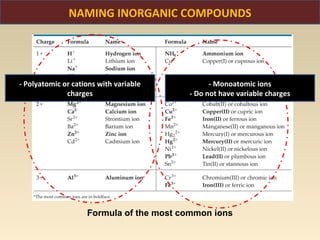
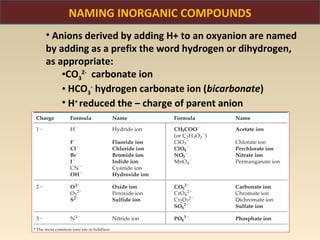
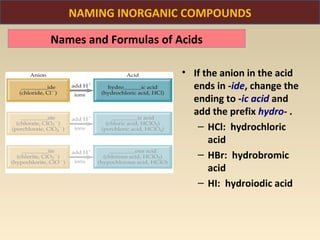
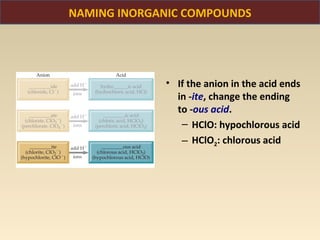
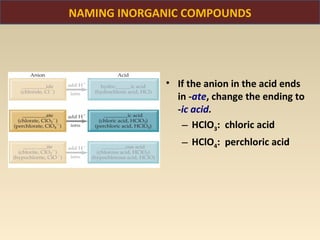
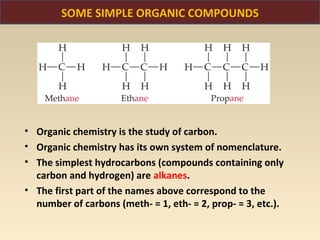
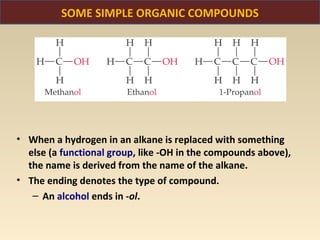
The document discusses the atomic theory of matter and the development of atomic structure models. It describes John Dalton's atomic theory which stated that elements are composed of atoms that are unique and atoms are neither created nor destroyed in chemical reactions. The discovery of the electron by J.J. Thompson and experiments by Robert Millikan and Ernest Rutherford helped develop the modern atomic structure model of a small, dense nucleus surrounded by electrons. The document also discusses isotopes, atomic numbers, mass numbers, and how the periodic table is arranged based on atomic structure.