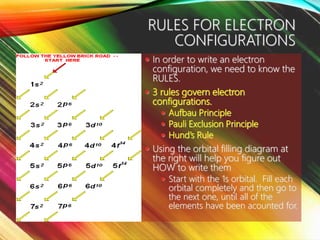
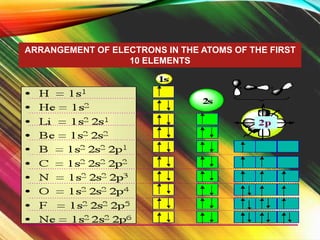
This document provides information about electron configuration. It begins by defining electron configuration as the arrangement of electrons in an atom's orbitals, which is described using quantum numbers. It then discusses the three main rules for writing electron configurations: 1) Aufbau principle, which states that electrons fill the lowest available energy levels first, 2) Pauli exclusion principle, which limits each orbital to two electrons of opposite spin, and 3) Hund's rule, which states that degenerate orbitals will fill with one electron each before pairing. The document provides examples of writing full and condensed electron configurations and drawing orbital diagrams for various elements. It includes an activity for students to practice these skills.











![SAMPLE PROBLEM Determining Electron Configuration
PLAN:
SOLUTION:
PROBLEM: Using the periodic table give the full and condensed electrons
configurations, partial orbital diagrams showing valence electrons,
and number of inner electrons for the following elements:
(a) potassium (K: Z = 19) (b) molybdenum (Mo: Z = 42) (c) lead (Pb: Z = 82)
Use the atomic number for the number of electrons and the periodic
table for the order of filling for electron orbitals. Condensed
configurations consist of the preceding noble gas and outer electrons.
(a) for K (Z = 19)
1s22s22p63s23p64s1
[Ar] 4s1
4s1
condensed configuration
partial orbital diagram
full configuration
There are 18 inner electrons.
3d 4p](https://image.slidesharecdn.com/electronconfigurationbyjbac-161111162940/85/Electron-Configuration-14-320.jpg)
![(b) for Mo (Z = 42)
1s22s22p63s23p64s23d104p65s14d5
[Kr] 5s14d5
(c) for Pb (Z = 82)
[Xe] 6s24f145d106p2
condensed configuration
partial orbital diagram
full configuration
5s1 4d5
condensed configuration
partial orbital diagram
full configuration 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p2
There are 36 inner electrons
and 6 valence electrons.
6s2 6p2
There are 78 inner electrons
and 4 valence electrons.
5p](https://image.slidesharecdn.com/electronconfigurationbyjbac-161111162940/85/Electron-Configuration-15-320.jpg)

![ASSESSMENT
1. Write the electron configurations (FULL &
ABBREVIATED) of each of the following atoms.
1. Scandium
2. Gallium
2. Determine what elements are denoted by the following
electron configurations:
3. [Kr] 5s24d105p3 ____________________
4. [Xe] 6s24f145d6 ____________________
c. Illustrate the orbital diagram for number #4.](https://image.slidesharecdn.com/electronconfigurationbyjbac-161111162940/85/Electron-Configuration-17-320.jpg)
